In this fifth phase of development, the contents of the Spanish Asthma Management Guidelines (GEMA), which include versions 5.0 and 5.1, have undergone a thorough review. The aim here is to set the main changes in context. These could be summarized as follows: DIAGNOSIS: new FENO cut-off and severity classification based on treatment needed to maintain control; INTERMITTENT ASTHMA: a more restrictive concept and treatment extended to include a glucocorticoid/adrenergic combination as needed; MILD ASTHMA: glucocorticoid/adrenergic therapy as needed as an alternative in case of low therapeutic adherence to conventional fixed-dose steroids; SEVERE ASTHMA: readjustment of phenotypes, incorporation of triple therapy in a single inhaler, and criteria for selection of a biologic in severe uncontrolled asthma; OTHERS: specific scoring in childhood asthma, incorporation of certain organizational aspects (care circuits, asthma units, telemedicine), new sections on COVID-19 and nasal polyposis.
La quinta fase de la Guía Española para el Manejo del Asma (GEMA) que incluye las versiones 5.0 y 5.1, ha efectuado una profunda revisión de su contenido. El presente texto tiene como objetivo contextualizar los principales cambios. Estos se podrían resumir en: DIAGNÓSTICO: nuevo punto de corte de FENO y clasificación de gravedad basada en el tratamiento necesario para mantener el control; ASMA INTERMITENTE: concepto más restrictivo y tratamiento ampliado a combinación de glucocorticoide/adrenérgico a demanda; ASMA LEVE: tratamiento con glucocorticoide/adrenérgico a demanda como alternativa si baja adhesión terapéutica a esteroide fijo clásico; ASMA GRAVE: reajuste de los fenotipos, incorporación de la triple terapia en un solo inhalador y criterios para la selección del fármaco biológico en asma grave no controlada; OTROS: puntualizaciones concretas en asma infantil, incorporación de determinados aspectos organizativos (flujos entre niveles asistenciales, unidades de asma, telemedicina), nuevas secciones de COVID-19 y de poliposis nasal.
The Spanish Asthma Management Guidelines (GEMA) has become the international reference clinical practice guideline (CPG) on asthma in the Spanish language. A total of 108 experts in asthma and related areas, selected by the 21 signatory scientific societies, associations, and foundations based in Spain, Portugal, and Latin America, participated in the latest versions of the fifth edition, 5.0 and 5.1. Since its inception more than 20 years ago, the guideline has fulfilled its aim to become a reference for healthcare professionals treating asthma patients, offering the best scientific evidence presented in a series of concise, simple, and practical recommendations.
Every 5 years, the guidelines are almost entirely renewed, with a revised format (new drafting and presentation) and new content (changes in concepts and approaches), while brief updates are produced annually to address recent developments in the literature. The previous edition, version 5.0, included important changes that were summarized in an article published in this journal1. This article supplements the previous document and its aims are 2-fold: to summarize the innovations introduced in the new revision, GEMA 5.1, and to contextualize the main modifications of the recently published fifth edition, some of which have generated some controversy (hence the title of this article). This is largely because the recommendations are not always in line with the proposals of other CPGs, particularly those of the international gold standard, the Global Initiative for Asthma (GINA)2. It is surprising that GEMA and GINA, both of which are based on studies and scientific evidence, put forward different recommendations. This is because the authors tm) interpretation of the available scientific and medical evidence reveals certain limitations in the state of the knowledge, insofar as the same evidence, interpreted differently, can give rise to different recommendations.
Conceptual changes and innovations are presented in 5 large blocks, as follows:
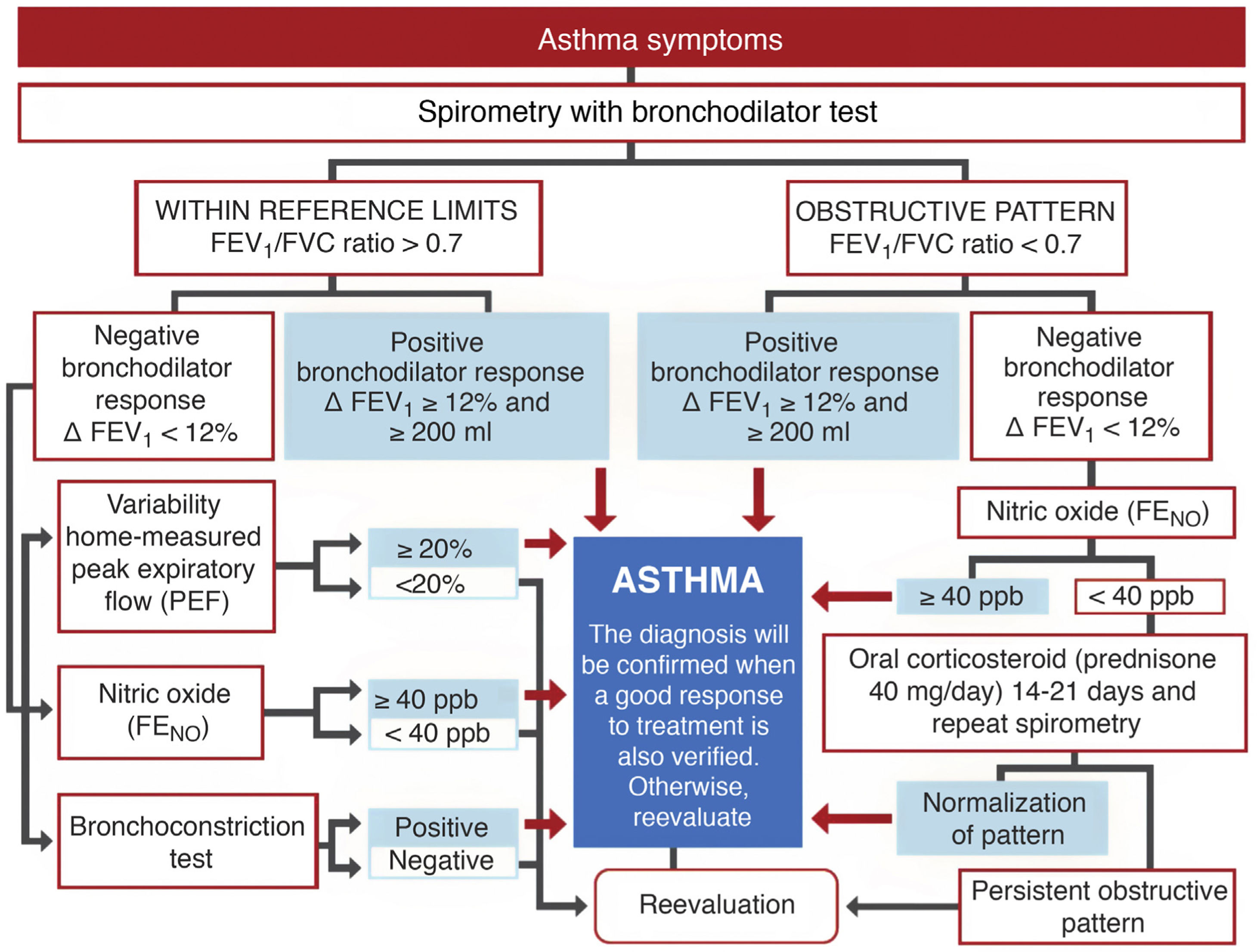
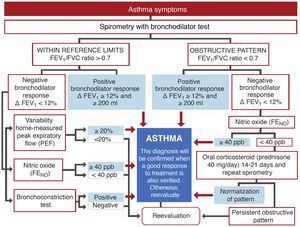
DiagnosisExhaled nitric oxide (FENO)Fig. 1 shows the standard GEMA algorithm for objectively confirming the diagnosis of asthma. It relies on the use of spirometry with a bronchodilator reversibility test that demonstrates significant reversible obstruction of the expiratory flow in a patient with symptoms of the disease. However, the examination is seldom diagnostic (most asthma patients have mild disease without pathological lung function), so in this setting, GEMA recommends using additional techniques that might increase diagnostic yield. Although all methods, including spirometry with a bronchodilator reversibility test, can give false positives, in a clinical context of high suspicion of asthma they can help the clinician reasonably assume the diagnosis of the disease. One of these methods is the measurement of FENO, which provides a simple, immediate, and non-invasive indirect measure of the bronchial inflammation typical of the T2 phenotype and partly related to eosinophilic inflammation3. The cut-off point for establishing the diagnosis is arbitrary: it is more demanding but safer to use a higher cut-off point, while a lower cut-off point is simpler but less reliable. This parameter has evolved over the years: it was initially set at 25 ppb, and then increased to 50. In the new GEMA, it has been reduced to 40, a level that most consensus documents and CPGs currently accept as the most reasonable4,5.
Severity classification Severity is an intrinsic feature of asthma, reflecting the intensity of pathophysiological abnormalities and response to treatment. In adult asthma, GEMA establishes 4 levels: intermittent, mild persistent, moderate persistent, and severe persistent. Establishing the level of severity is an essential step in clinical practice, as the nature, prognosis, and treatment of the disease differ widely between the different levels. Indeed, intermittent asthma and severe asthma are so dissimilar that they could almost be two different entities. Methods to establish severity have also evolved over time. The new GEMA, like other CPGs, advises against determining severity at the start of therapy in favor of waiting until the patient is receiving adequate maintenance treatment that has been assessed and adjusted over a reasonable period of time. Furthermore, severity is not static and can vary over time.
In short, severity is related to the amount of medication a patient needs to maintain control and prevent exacerbations6, as shown in Table 1. For example, if an individual requires the recommended maintenance treatment at step 3 to maintain control, he or she will have moderate persistent asthma.
Intermittent asthmaConcept. Intermittent asthma, the first level of disease severity, remains a topic of debate. This level would include forms of asthma that are predominantly asymptomatic, but with sporadic manifestations. Some authors and some CPGs, such as GINA2, do not accept this classification and include these occasional forms of asthma under mild persistent asthma. Ultimately, the symptom frequency cut-off point to establish the possible difference between intermittent and mild asthma is arbitrary. Furthermore, the standard use of exclusively on-demand inhaled short-acting β2 agonists (SABA) to treat this stage may contribute to the abuse of such therapy to the detriment of appropriate anti-inflammatory treatment with inhaled corticosteroids (ICS) when the need for SABA increases. However, denying its existence would require persistent maintenance treatment in patients with very occasional asthma symptoms, such as individuals with allergic asthma associated with pollens who may be completely asymptomatic between seasons, or in children with very sporadic symptoms in whom prolonged therapy with ICS is inadvisable.
On the basis of these arguments, the majority of the members of the GEMA Executive Committee decided, after intense debate, to maintain this level, but they have tightened the criteria. In these new versions of GEMA, intermittent asthma is defined as symptoms or need for rescue medication occurring twice a month or less, with no exacerbations and lung function within the reference values, whereas in past editions it was twice a week or less, as shown in Table 2.
Classification of asthma control in adults.
| Well controlled (all of the following) | Partially controlled (any measured in any week) | Poorly controlled | |
|---|---|---|---|
| No daytime symptoms | None or ≤ 2 days per month | > 2 days a month | ≥ 3 characteristics of partially controlled asthma |
| Limitation of activities | None | Any | |
| Nocturnal symptoms/arousals | None | Any | |
| Need for reliever medication (rescue) (short-acting β2 adrenergic agonist) | None or ≤ 2 days per month | > 2 days a month | |
| Lung function | |||
| FEV1 | ≥ 80% predicted value or z-score (-1.64) | < 80% of predicted value or z-score (-1.64) | |
| PEF | ≥ 80% of best personal value | < 80% of best personal value | |
| Exacerbations | None | ≥ 1/year | ≥ 1 in any week |
FEV1: forced expiratory volume in 1 second; PEF: Peak expiratory flow or maximum expiratory flow.
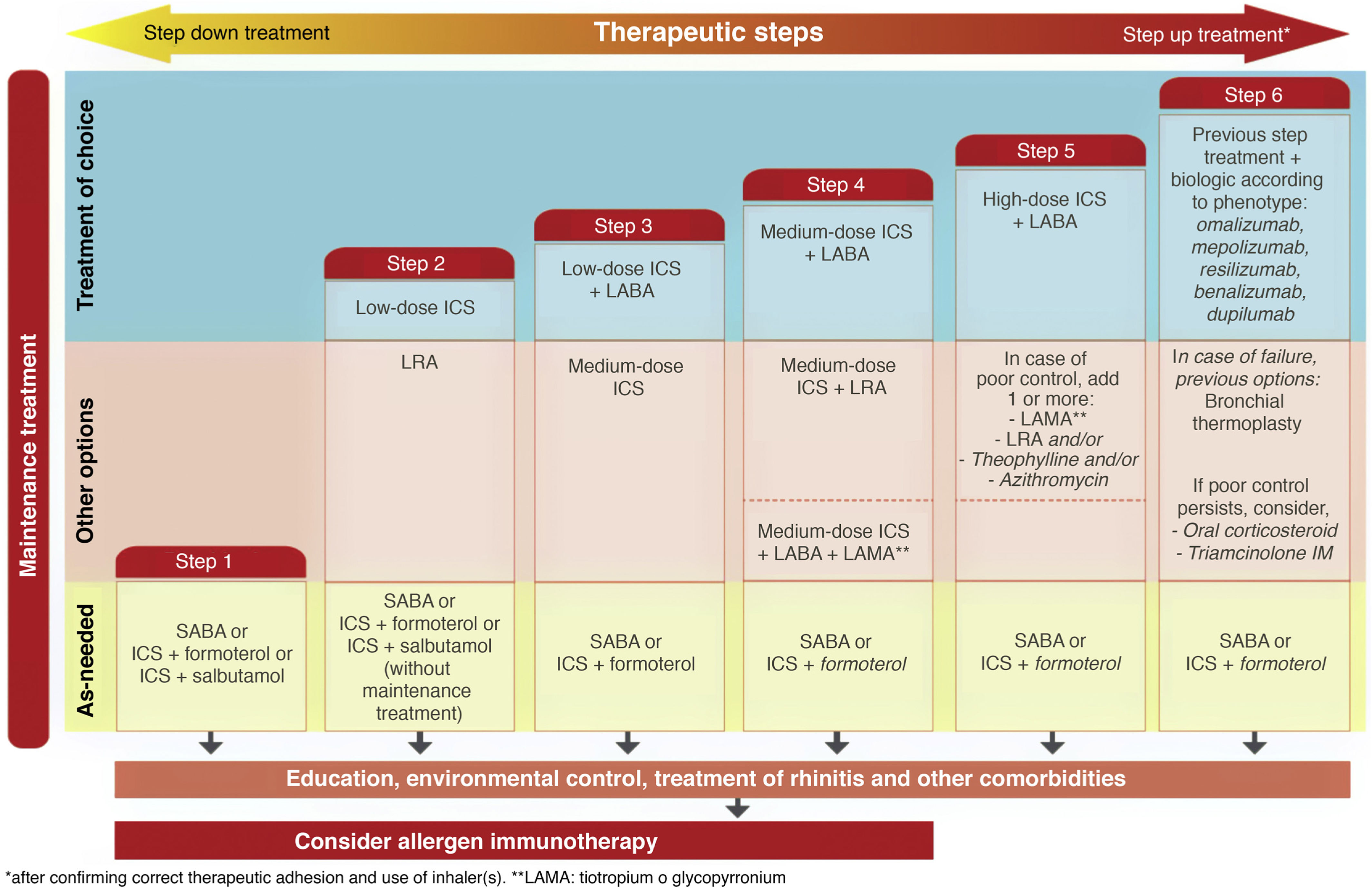
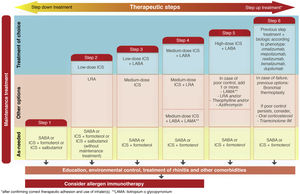
Treatment. In this fifth edition of GEMA, the conceptual change in the definition of intermittent asthma was also accompanied by a change in treatment. As previously mentioned, this disease level has traditionally been treated with on-demand SABA administered according to the need perceived by the patient in the presence of asthma symptoms. However, this strategy could inadvertently promote the overuse of SABA and the underuse of ICS7. Several studies have shown an overuse of SABA that is associated with greater asthma morbidity and mortality8,9. In contrast, some studies reported the efficacy and safety at this level of on-demand administration of a low-dose ICS (budesonide or beclomethasone) combined with formoterol or salbutamol in a single inhaler10,11.
The new versions of GEMA, then, propose treating patients with intermittent asthma with 1 of the following 3 drugs regimens administered on demand (Fig. 2): SABA; or combination of ICS (low-dose)/formoterol; or combination of ICS (low-dose)/salbutamol, depending on the patient's clinical characteristics. This allows clinicians to tailor treatment to the needs of each patient. However, additional techniques (spirometry, FENO) should be used to rule out more severe forms of the disease in patients who present few symptoms and consequently will need more vigorous maintenance treatments.
Mild asthmaOne of the most controversial issues at present is undoubtedly the best therapeutic option for the treatment of patients with mild asthma. As the majority of asthma patients present mild disease, this is a major concern due to the economic consequences to both payers (often public health systems) and manufacturers/retailers (pharmaceutical industry). An international group of experts, consisting mostly of leaders of the current GINA consensus, recently reported that asthma control in most patients remained poor despite increasingly effective and safe treatments, and in response promoted a therapeutic paradigm shift7.
To demonstrate the effectiveness of a new therapeutic strategy in mild asthma maintenance, the as-needed administration of the budesonide/formoterol combination was compared with the standard fixed daily ICS (low-dose) treatment in 2 clinical trials, known as the SYGMA studies. The effect of both strategies on several variables was studied, including exacerbation prevention (main outcome), control, lung function, and quality of life12,13. Both strategies showed a similar ability to prevent exacerbations, but in general, patients who received as-needed budesonide/formoterol had significantly lower levels of control, lung function, and quality of life. Hypothesizing that therapeutic adherence to fixed-dose ICS is low among patients with mild asthma, particularly young people, and that the as-needed administration of budesonide/formoterol could provide greater therapeutic efficacy, two “real-life” studies, Novel START14 and PRACTICAL15, were subsequently conducted using similar variables and assumptions to the SYGMA studies. However, questions have been raised about both studies, suggesting that the designs are suboptimal (cases with under-treated moderate asthma were included), the therapeutic adherence rates are surprisingly high for real-life scenarios (76%), and the outcomes are disparate.
While GINA2 accepts this as sufficient evidence for giving budesonide/formoterol the same level of recommendation as fixed-dose ICS as first-line treatment of mild asthma, the European health authorities do not approve this strategy. They believe that the evidence shows the opposite, i.e., that the new option is inferior, and for the moment, this combination has not been approved in the Summary of Product Characteristics for on-demand administration in mild or intermittent asthma. GEMA, on the other hand, positions itself somewhere between these 2 extremes (Fig. 2), and continues to recommend daily, long-term administration of low-dose ICS as the treatment of choice in mild persistent asthma (step 2) (recommendation 3.4). However, as an alternative, the as-needed use of budesonide/formoterol, beclomethasone/formoterol, or beclomethasone/salbutamol could be considered in patients with low therapeutic adherence in whom a specific corrective educational intervention has failed (recommendation 3.5). Nevertheless, as mentioned above, this strategy is not approved in the SmPC of these products and its cost-effectiveness is unknown.
The question remains open and much remains to be clarified about this potential new strategy: Is there a candidate patient profile? And a non-candidate profile? Is underlying bronchial inflammation sufficiently treated? How do these patients progress clinically in the medium and long term? More evidence, including other studies, is needed, and a well-designed study in proper real-life conditions is called for, if we are to definitively establish the effectiveness of this strategy.
Severe asthmaThe emergence of new treatments targeting certain variants of severe asthma has significantly increased our knowledge of this entity, and this section of the guideline has grown proportionately.
Phenotypes. In line with the practical approach that characterizes the latest editions of GEMA, the need to establish phenotypes has been restricted to severe asthma alone. This is primarily because obtaining this information at lower severity levels increases complexity to a probably unnecessary degree (an important consideration in the primary care setting), especially when less severe clinical forms respond well to standard treatment.
In patients with severe uncontrolled asthma (SUA), however, establishing the asthma phenotype is part of the diagnostic process, as different treatment regimens may be required and prognoses may vary. To this end, clinical (age, onset of symptoms, presence of allergic symptoms, upper respiratory tract involvement, presence of aspirin-exacerbated respiratory disease [AERD]), functional (spirometry, FENO), and laboratory (eosinophils in blood and sputum, total immunoglobulin E [IgE], induced sputum neutrophils) parameters are determined16•18. This information identifies 2 types of inflammatory pattern: T2-high and T2-low, according to which SUA can be divided into 3 phenotypes, T2-allergic, T2-eosinophilic and non-T2 (Table 3), although it is common to observe some degree of overlap between the two T2 phenotypes. The former “severe obesity-related asthma” phenotype has been dispensed with, since obesity (that is significantly more frequent in severe asthma) is probably more likely to be a comorbidity than a specific phenotype.
Severe asthma phenotypes.
| Phenotypes | Clinical characteristics | Biomarkers | Treatment |
|---|---|---|---|
| Allergic (T2) | Allergic symptoms + allergen sensitization (skin prick test and/or specific IgE) | Specific IgE | Corticosteroids |
| Th2 cytokines | Omalizumab | ||
| Periostin | Anti IL-5/IL-5R (mepoluzimab, reslizumab, benralizumab) | ||
| Eosinophils and neutrophils in sputum | Dupilumab | ||
| Eosinophilic (T2) | Chronic rhinosinusitis/nasal polyposis | Eosinophils in blood and IL-5 in sputum | LRA |
| AERD | Cysteinyl leukotrienes | Anti IL-5/IL-5R (mepoluzimab, reslizumab, benralizumab) | |
| Corticosteroid-dependent or corticosteroid-resistant | Dupilumab | ||
| Non-T2 | Lower FEV1 | Neutrophilic or paucigranulocytic sputum | Azithromycin |
| Increased air trapping | TH17 activation | ||
| History of smoking | IL-8 |
AERD: acetylsalicylic acid-exacerbated respiratory disease; FEV1: forced expiratory volume in 1 second; IgE: immunoglobulin E; IL: interleukin; LRA: leukotriene receptor antagonist.
Triple therapy in a single inhaler. Probably the most notable innovation of GEMA 5.1 is the addition of the combination of an ICS, a long-acting β2 adrenergic agonist (LABA), and a long-acting antimuscarinic (LAMA) in a single inhaler to the therapeutic arsenal for asthma (Fig. 2). Although tiotropium was used in the past as the only LAMA accepted for the treatment of severe asthma, along with a combination of ICS/LABA, it was administered in separate inhaler (Respimat¨r)). Recent clinical trials have shown that a triple combination in a single inhaler, using glycopyrronium (pressurized cartridge or single-dose dry powder) as the LAMA, improved lung function and reduced exacerbations in patients who were under-controlled with an ICS/LABA, particularly in individuals with airflow obstruction (FEV1/postbronchodilator FVC ratio ≤ 70%)19•21.
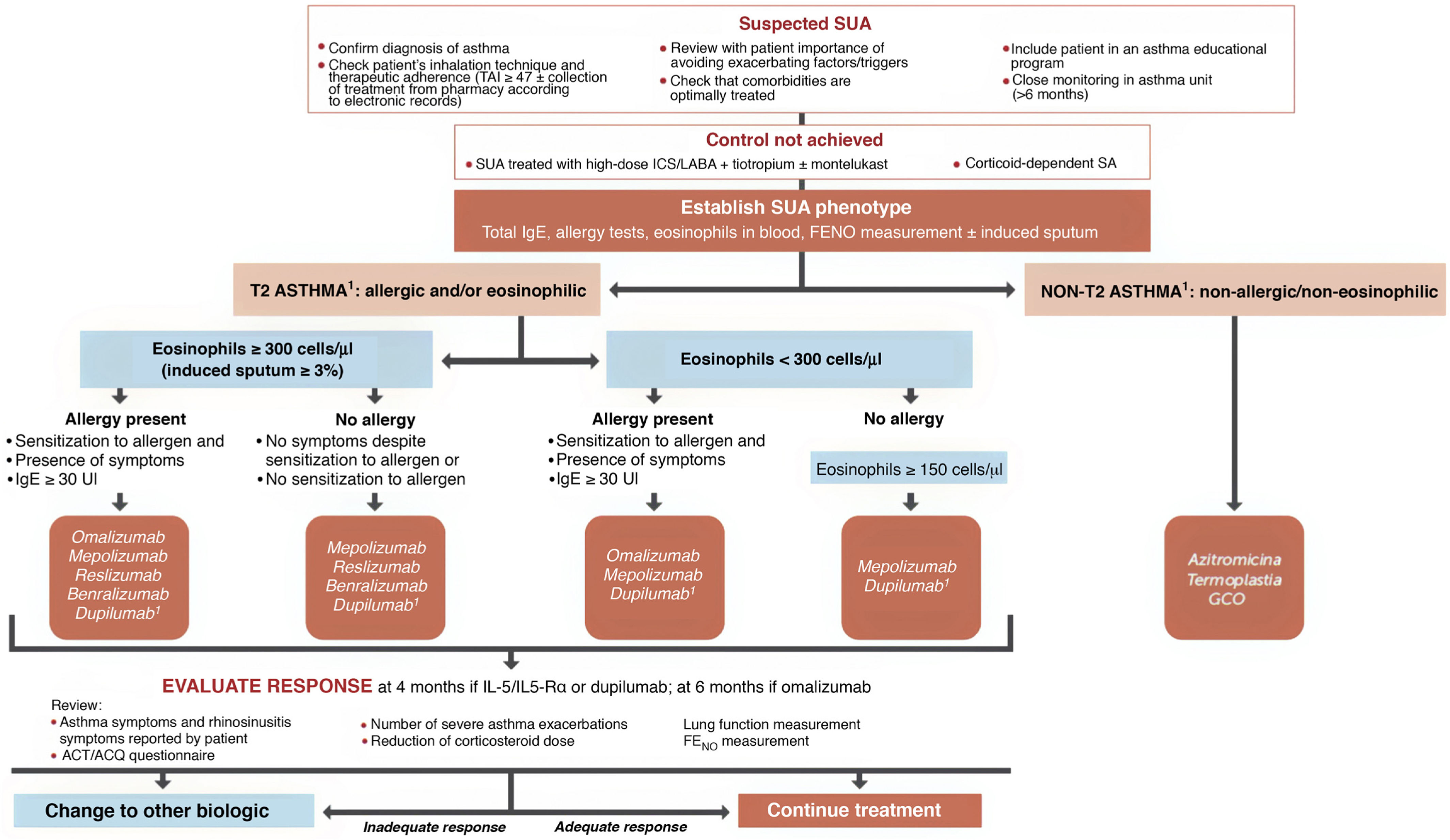
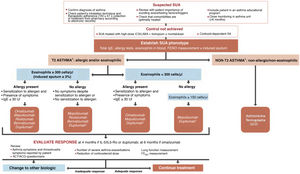
Selecting a biologic for the treatment of severe uncontrolled asthma. One of the highlights of this fifth edition of GEMA was the incorporation of recently marketed biological drugs for the treatment of SUA. The mechanisms of action of the biologics approved by the EMA and listed in this guideline for the treatment of T2 SUA are as follows: anti-IgE (omalizumab), anti-interleukin 5/interleukin 5Rα (IL-5/IL-5Rα) (mepolizumab, reslizumab and benralizumab), and anti-IL13/IL4 (dupilumab).
The algorithm proposed for selecting a biological drug is particularly novel compared with other CPGs (Fig. 3) and based largely on the previous Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) consensus on severe asthma22. After the patient tm)s asthma phenotype is established as described above, paying particular attention to the results of total IgE, skin allergy tests, blood eosinophils (or induced sputum), and FENO, asthma is classified as T2 or non-T2. In the case of T2 asthma, the eosinophil count (greater than or less than 300 µL) places the patient in one of two large groups: eosinophilic T2 asthma or non-eosinophilic T2 asthma. Subsequently, each patient is assigned to 1 of the 4 groups indicated in the algorithm depending on whether they have allergic eosinophilia, based on the result of skin allergy tests, total IgE, and medical history suggestive of allergy. An initial therapeutic option is then proposed, which, in case of difficulties (intolerance, allergies, administration problems, etc.) or treatment failure (total or partial), may be changed to any of the others proposed in the group.
Treatment of severe uncontrolled asthma according to inflammatory phenotype.
ACT: Asthma Control Test; ACQ: Asthma Control Questionnaire; ICS: inhaled corticosteroids; LABA: long-acting bronchodilators; OCS: oral corticosteroids; SA: severe asthma; SUA: severe uncontrolled asthma; TAI: Test of Adherence to Inhalers.
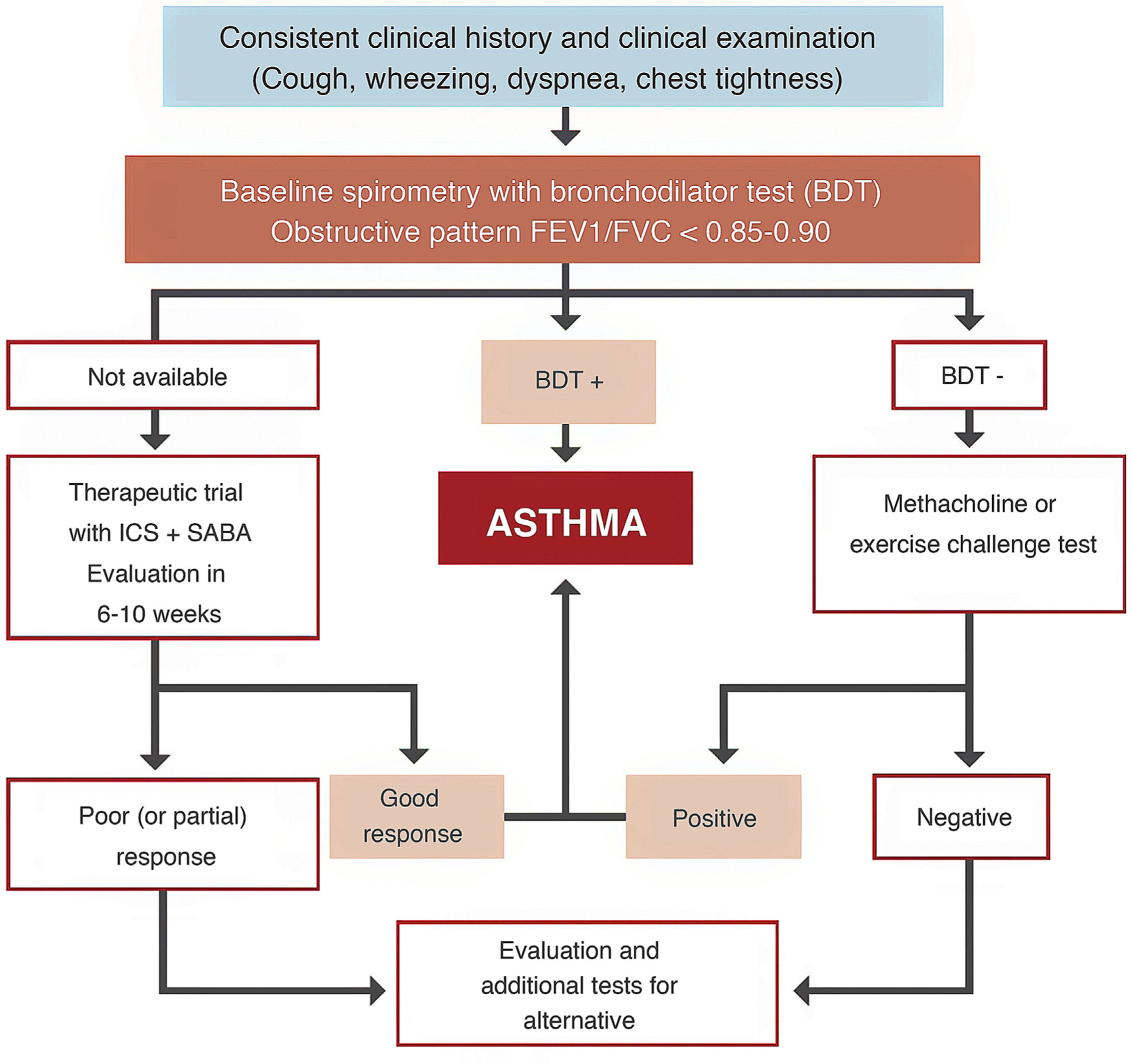
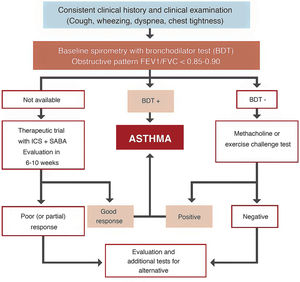
Space restrictions mean that we cannot describe in detail all the modifications introduced in this new GEMA, but among the wide-ranging selection of topics addressed in this fifth edition, we must highlight the inclusion of some local multidisciplinary consensus documents developed to improve patient flow between care levels and with emergency services23,24. We should also mention the accreditation of asthma units by SEPAR and the Spanish Society of Allergy and Clinical Immunology (SEAIC)25, and the role of telemedicine in the follow-up of asthma patients. The inclusion of new sections, such as rhinosinusitis and nasal polyposis (6.5) in chapter 6 on asthma-associated rhinitis and, in the GEMA 5.1 revision, allergic bronchopulmonary aspergillosis (8.8) in chapter 8 on special circumstances, also deserves a mention. A simplified written action plan is proposed for use in the short or minimal educational interventions that are recommended if a formal or classic asthma education program cannot be implemented26,27. The multiple changes introduced in the various sections addressing childhood asthma should also be highlighted, including the proposed diagnostic algorithm (Fig. 4), the role of FENO in monitoring, some remarks about treatment in general, and the prescription of mepolizumab in the section on severe asthma.
Furthermore, given the current SARS-CoV-2 coronavirus pandemic and the multiple publications evaluating the impact of COVID-19 on asthma and vice versa, the guideline would be incomplete without a section to discuss these issues. In short, it could be stated (provisionally) that according to the current available evidence, non-severe asthma is not associated with a higher probability of developing COVID-19, nor is it associated with more severe infection or higher morbidity28. However, the data from patients with severe asthma are more equivocal, with some series showing higher infection rates and morbidity and mortality29, and others not30. Recent studies on large case samples seem to suggest that treatment with ICS may offer some protection against infection31. A practical table of possible drug interactions between drugs used to treat infection and asthma has also been included32.
Finally, we would like to highlight the balanced position that GEMA takes regarding certain controversial issues, a position in which we feel comfortable. The guideline must reach beyond the debates, in which we all participate, and be based on evidence that must be evaluated with the greatest possible impartiality, without being influenced by beliefs, other CPGs, and external pressures. At the end of the day, our hope for GEMA is that it offers a simple but rigorous recommendation document that is easy for healthcare professionals to apply in the care of their asthma patients.
Conflict of interestsThe editors of the guideline state that they have received honoraria in the past two years for their participation in meetings, congresses or research work organized by the following pharmaceutical industries: Astrazeneca, Chiesi, GSK, Novartis, ALK, Gebro Pharma, Menarini, Mundipharma, Pfizer, Sanofi Genzyme, and Faes Farma.