Epidemiological data suggest that sleep apnea–hypopnea syndrome (SAHS) is independently associated with the development of insulin resistance and glucose intolerance. Moreover, despite significant methodological limitations, some studies report a high prevalence of SAHS in patients with type 2 diabetes mellitus (DM2). A recent meta-analysis shows that moderate–severe SAHS is associated with an increased risk of DM2 (relative risk=1.63 [09–2.45]), compared to the absence of apneas and hypopneas.
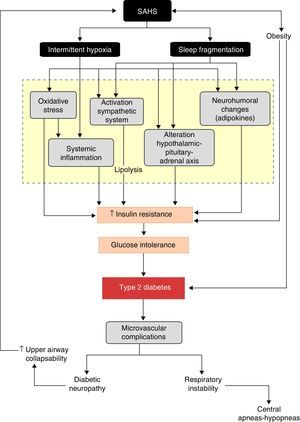
Common alterations in various pathogenic pathways add biological plausibility to this relationship. Intermittent hypoxia and sleep fragmentation, caused by successive apnea–hypopnea episodes, induce several intermediate disorders, such as activation of the sympathetic nervous system, oxidative stress, systemic inflammation, alterations in appetite-regulating hormones and activation of the hypothalamic–pituitary–adrenal axis which, in turn, favor the development of insulin resistance, its progression to glucose intolerance and, ultimately, to DM2.
Concomitant SAHS seems to increase DM2 severity, since it worsens glycemic control and enhances the effects of atherosclerosis on the development of macrovascular complications. Furthermore, SAHS may be associated with the development of microvascular complications: retinopathy, nephropathy or diabetic neuropathy in particular. Data are still scant, but it seems that DM2 may also worsen SAHS progression, by increasing the collapsibility of the upper airway and the development of central apneas and hypopneas.
Diversos datos epidemiológicos muestran que el síndrome de apneas-hipopneas del sueño (SAHS) se relaciona independientemente con el desarrollo de resistencia a la insulina e intolerancia a la glucosa. Además, y pese a la existencia de notables limitaciones metodológicas, algunos estudios refieren una elevada prevalencia de SAHS en pacientes con diabetes tipo 2 (DM2). Un reciente metaanálisis muestra que el SAHS moderado-grave se asocia a un mayor riesgo de DM2 (riesgo relativo=1,63 [09–2,45]), en relación con la ausencia de apneas-hipopneas.
La existencia de alteraciones comunes de diversas vías patogénicas le proporciona plausibilidad biológica a esta relación. La hipoxia intermitente y la fragmentación del sueño, originadas por la sucesión de episodios de apneas-hipopneas, inducen diversos trastornos intermedios, como la activación del sistema nervioso simpático, el estrés oxidativo, la inflamación sistémica, alteraciones en las hormonas reguladoras del apetito y activación del eje hipotálamo-hipófiso-suprarrenal, que favorecen el desarrollo de resistencia a la insulina, así como su progresión a intolerancia a la glucosa y, en última instancia, a DM2.
La coexistencia del SAHS parece agravar la evolución de la DM2, al empeorar el control glucémico y potenciar el efecto de la aterosclerosis en el desarrollo de complicaciones macrovasculares. Además, el SAHS podría asociarse al desarrollo de complicaciones microvasculares, particularmente la retinopatía, nefropatía o neuropatía diabéticas. Aunque todavía escasos, algunos datos sugieren que la DM2 también podría empeorar la evolución del SAHS, al favorecer la colapsabilidad de la vía aérea superior y potenciar la aparición de apneas-hipopneas centrales.
Sleep apnea–hypopnea syndrome (SAHS) is a public health problem of the first order,1 due to its high prevalence2,3 and marked morbidity and mortality,4–6 having been linked to traffic accidents,7,8 cardiovascular complications9–25 and, more recently, neoplastic diseases.26 One increasingly interesting aspect is its relationship with metabolic disorders, specifically type 2 diabetes (DM2).
Diabetes mellitus is a global epidemic.27 There are currently 382 million diabetics worldwide, a figure which is estimated to reach 592 million in 2035.28 DM2, which accounts for 90%–95% of all diabetes cases,29 is a complex metabolic disorder in which the interaction of genetic and environmental factors causes a deficiency in both insulin secretion and insulin sensitivity.
In healthy subjects, glucose homeostasis is reached by controlling glucose production by the liver (gluconeogenesis) and its use by insulin-dependent tissues, such as muscle and fat, and by non-insulin-dependent tissues, such as the brain.30 Glucose uptake by peripheral tissues is regulated by insulin, which is produced by pancreatic islet β-cells, both constitutionally and in response to an increase in blood glucose. Insulin also suppresses hepatic gluconeogenesis and adipose tissue lipolysis. The biological response of insulin target tissues (insulin sensitivity) has several physiological determinants, particularly the amount of fatty tissue. A decrease in the peripheral response to insulin (insulin resistance) reduces tissue glucose uptake and leads to glucose intolerance.31 Failure by β-cells to secrete sufficient insulin to overcome insulin resistance results in DM2.32
The presence of common risk factors, as well as the involvement of some shared pathogenic pathways, explains the potential relationship between both entities. Therefore, the aims of this review were to analyze clinical–epidemiological data that support the existence of a possible relationship between SAHS and DM2, to evaluate the pathogenic mechanisms potentially involved, and to assess the prognostic impact of this relationship.
Clinical and Epidemiological EvidenceAvailable evidence suggests that SAHS may alter glucose metabolism, progressing from an increase in glucose resistance to glucose intolerance, poorer metabolic control of blood glucose and, ultimately, DM2.
Despite the controversial findings in 1 preliminary study,33 it appears that SAHS increases insulin resistance independently of obesity. Ip et al.34 analyzed the relationship between SAHS and insulin resistance in 270 non-diabetic subjects, evaluated by the homeostatic model assessment (HOMA) index. They found that patients with SAHS had a higher HOMA index, i.e. they had more insulin resistance than patients without SAHS. Furthermore, both obesity and the apnea–hypopnea index (AHI) and minimum blood oxygen saturation (SaO2) were independent determinants of insulin resistance.
The effect of SAHS on insulin resistance appears to be not only independent of obesity, but also of body fat distribution and sex. In 194 non-diabetic patients with SAHS, the HOMA index was higher in patients with severe SAHS, and was independently associated with body mass index (BMI), AHI and plasma adiponectin concentration.35 Similarly, in a population sample of 400 women aged between 20 and 70 years, Theorell-Haglöw et al.36 showed that insulin sensitivity was lower in patients with severe SAHS, associating it with minimum SaO2, when adjusting for age, waist–hip ratio, physical activity, smoking and alcohol consumption.36
The influence of SAHS on the development of insulin resistance has also been confirmed in longitudinal studies, such as that conducted by Lindberg et al.,37 which analyzed its effect on carbohydrate metabolism in a population sample of men without diabetes. They found that the desaturation index, AHI, and minimum SaO2 were independently related with changes in the HOMA index during patient follow-up. Studies have also tried to adjust for the possible influence of obesity on the SAHS–insulin resistance association, evaluating it in lean individuals. Pamidi et al.38 reported that lean men with SAHS have 27% lower insulin sensitivity and 37% higher insulin secretion than controls matched by age, BMI, family history and exercise levels. Similar results were found in another study in lean men conducted in China.39 Ultimately, the available evidence appears to show that SAHS is independently related with the development of insulin resistance, and therefore increases the risk of diabetes.
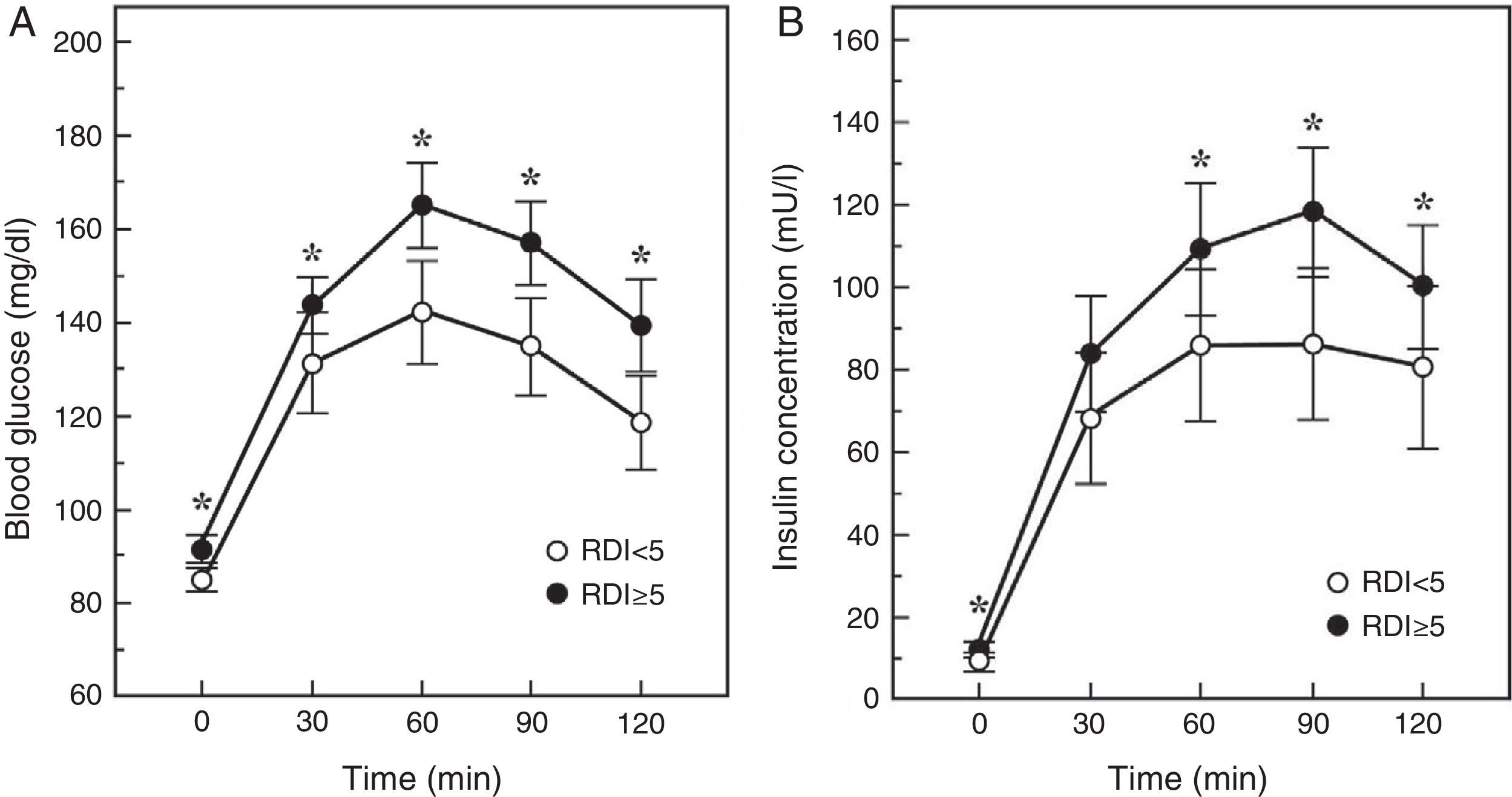
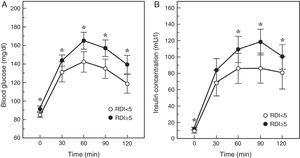
An intermediate step in the natural history of the SAHS-diabetes relationship is the decrease in glucose tolerance. In fact, in 150 healthy men with neither diabetes nor cardiopulmonary disease, an AHI≥5 was found to double the risk of glucose intolerance, depending mainly on the severity of the nocturnal desaturation.40 Similarly, the Cleveland Family Study found that the principal determinant of glucose intolerance was time with SaO2<90% (CT90), which was present in 32% of subjects with SAHS. Individuals with a CT90≥2% had 2.33 (95% CI: 1.38–3.94) times the risk of glucose intolerance.41 In the Sleep Heart Health Study, the largest epidemiological study conducted to date, investigators assessed the presence of SAHS by polysomnography, and measured baseline glucose and insulin levels and then again following an oral glucose tolerance test (OGTT). They found that once adjusted for age, BMI, waist circumference, race, sex and smoking habits, subjects with an AHI≥15 had odds ratios of 1.46 and 1.44 for high baseline or post-OGTT blood glucose levels.42 More recently, Cizza et al.43 reported that 44% of sleep-deprived obese adults had glucose intolerance, and that more severe SAHS was related with higher baseline blood glucose and insulin levels, and with a poorer response to the OGTT (Fig. 1).
Blood glucose and insulin response to an oral glucose tolerance test in patients with (RDI or respiratory disturbance index≥5) or without SAHS. Adapted from Cizza et al.43
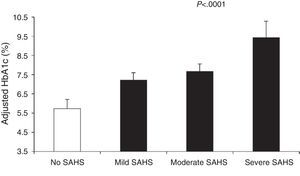
Finally, there is also evidence directly linking SAHS with poorer metabolic glucose control.36,44–50 In patients with no known diabetes, a dose-response relationship was observed between AHI and the percentage of poor blood glucose control, defined as a glycated hemoglobin level (HbA1c)>6%, which rose to 34.2% when the AHI was greater than 50.51 With respect to subjects with an AHI<5, the presence of an AHI of 15–30 or ≥50 had an adjusted odds ratio for poor blood glucose control of 1.80 (1.19–2.72) and 2.96 (1.58–5.54), respectively. Moreover, hypoxemia during sleep was also related with HbA1c>6%.51
In short, the data from various clinical and epidemiological studies support an association between SAHS and impaired glucose homeostasis, which exposes these patients to a higher risk of developing diabetes.
Prevalence of Sleep Apnea–Hypopnea Syndrome in Patients With Type 2 DiabetesDespite notable methodological limitations, several studies report a higher prevalence of SAHS in patients with DM2 than in the general population.52–57 Unfortunately, as yet, few studies have used universally accepted diagnostic methods such as polygraphy and polysomnography. The main evidence comes from various sub-analyses in a multiethnic cohort of subjects aged over 40 years with no known cardiovascular disease, enrolled in the Sleep Heart Health Study. In a sample of 4991 participants, it was found that the Respiratory Disturbance Index (RDI) was associated with the presence of diabetes, as well as age, BMI, waist–hip ratio, hypertension and lipid levels.58 In another series of 5874 subjects, differences were noted in the RDI and the CT90 between diabetic and non-diabetic patients.59 When the presence of SAHS was measured in the same cohort using polysomnography, it was found in 58% of the group of patients with DM2.60 Two subsequent studies also identified high prevalence of SAHS in patients with DM2: in one, up to 62% of patients hospitalized for poorly controlled DM2 had SAHS (34% mild, 19% moderate and 10% severe),55 and in the other, SAHS was found in 86% of obese diabetics.56
Prevalence of Type 2 Diabetes in Patients With Sleep Apnea–Hypopnea SyndromeThe prevalence of DM2 in patients with SAHS ranges from 15% to 30%, depending on the study population, definition of severity of SAHS, and the diagnostic methods used.61,62 The Wisconsin Sleep Cohort study detected a higher prevalence of DM2 in patients with SAHS (14.7% vs 2.8%), with an adjusted odds ratio of 2.3 (1.28–4.11) for an AHI≥15 with respect to an AHI<5.48 In contrast, a multiethnic study of 1008 patients with DM2, predominantly Hispanic and African-American, gave negative results. Although the prevalence of DM2 was also higher in subjects with SAHS than in nonapneic subjects (30.1% vs 18.6%), giving an odds ratio of 1.8 (1.3–2.6), this association was not significant when controlling for various confounding factors.63 However, another cross-sectional study conducted in a much larger sample (14,440 subjects in the Hispanic Community Health Study/Study of Latinos) confirmed that moderate SAHS is associated with both glucose intolerance (odds ratio=1.7; 95% CI: 1.3–2.1) and diabetes (2.3; 1.8–2.9).64
In addition to the relationship between SAHS and DM2 shown in these cross-sectional studies, some longitudinal studies indicate that the existence of apneas–hypopneas may lead to the development of diabetes. Marshall et al.61 provided the first evidence that moderate–severe SAHS is a risk factor for diabetes. After a 4-year follow-up of an Australian population cohort, they found that 20% of patients with moderate–severe SAHS had been diagnosed with DM2, showing that SAHS is an independent risk factor for incident diabetes (adjusted hazard ratio=13.45; 95% CI: 1.59–114.11). Lindberg et al.37 reported that this effect is not confined to the moderate–severe forms. In an 11-year follow-up of a population sample of non-diabetic men, they found that, after adjusting for confounders, a desaturation index>5 was a predictor for the onset of diabetes (4.4; 1.1–18.1). Another observational study of a cohort of 1233 patients in the Veteran Affairs Connecticut Health Care System confirmed the role of SAHS as a risk factor for DM2.65 After adjusting for age, sex, race, baseline fasting blood glucose, BMI and weight change, an independent association was detected between SAHS and incident diabetes (1.43; 1.10–1.86). A 16-year follow-up of a small cohort of middle-aged women referred to a sleep clinic also found that SAHS predicted the appearance of new-onset diabetes in females.62
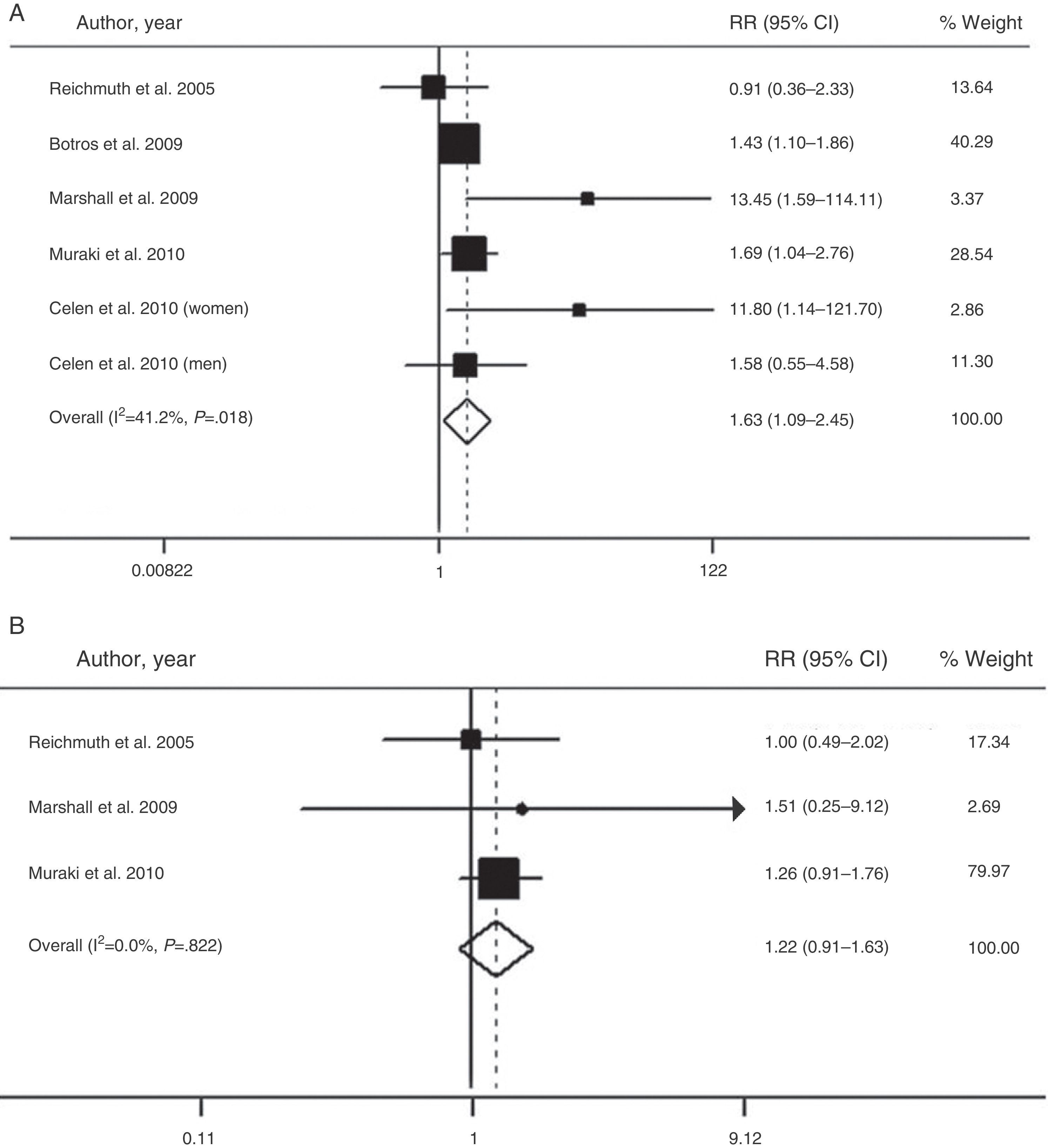
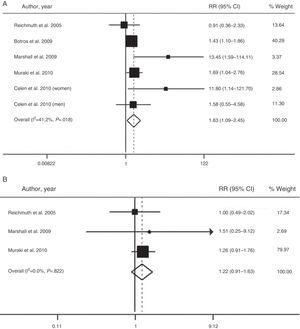
The overall value of the available evidence has recently been evaluated in a meta-analysis of six prospective cohort studies with a total of 5953 participants and a follow-up period of 2.7–16 years.66 This analysis confirmed that moderate–severe SAHS is associated with a higher risk of diabetes (relative risk=1.63; 95% CI: 1.09–2.45), when compared with the absence of SAHS (Fig. 2). However, patients with mild SAHS did not show a higher risk than nonapneic patients (1.22; 0.91–1.63).66
Risk of the association between moderate-severe (A) and mild SAHS (B) and type 2 diabetes. CI: confidence interval; RR: relative risk. Adapted with the permission of the Asian Pacific Society of Respirology. Copyright© 2012 Asian Pacific Society of Respirology. Wang et al.66
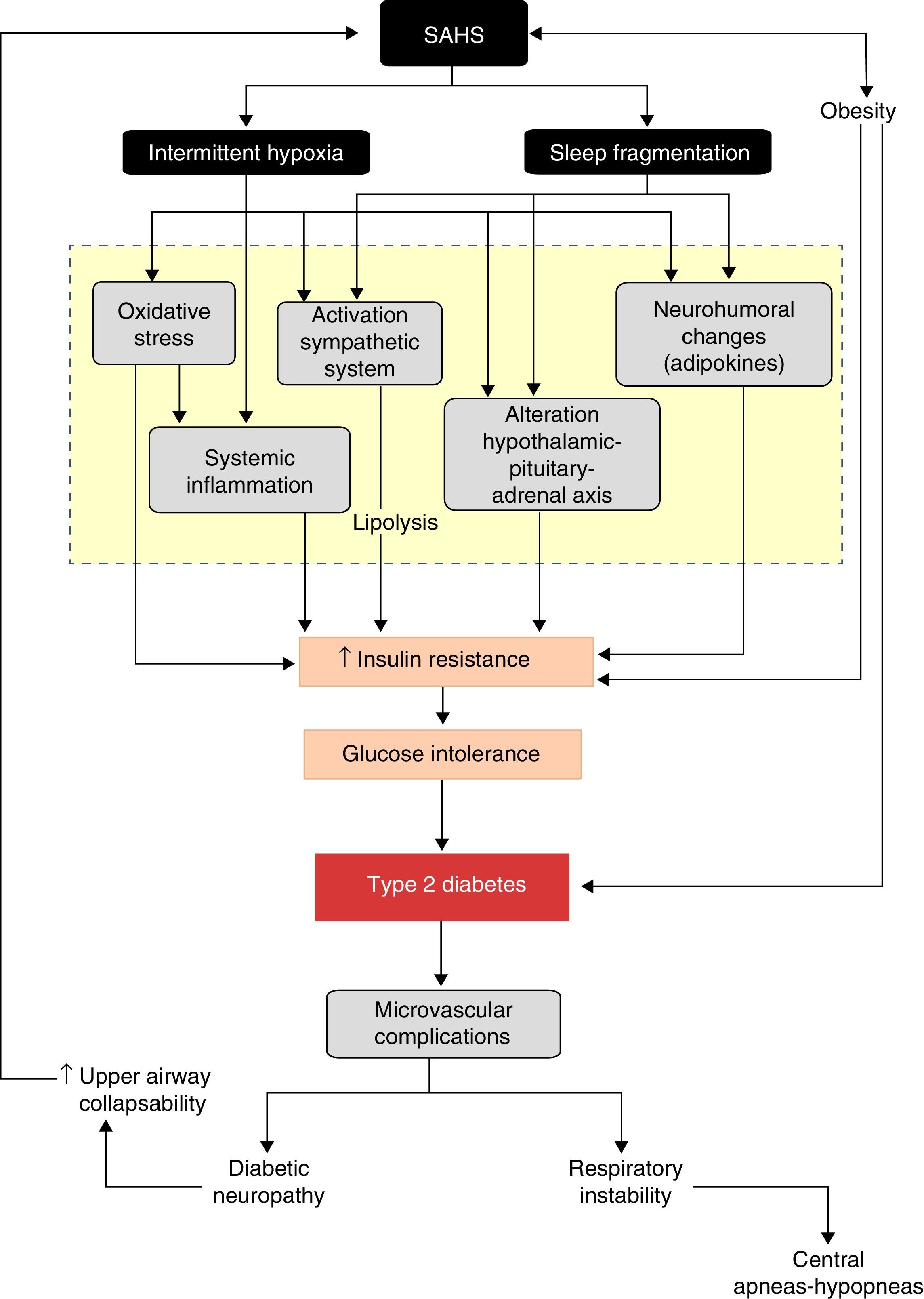
A better understanding of the effects of physiopathological disorders caused by apneas–hypopneas on glucose metabolism lends greater plausibility to the SAHS-DM2 relationship and can be summarized as follows: SAHS activates 2 triggering mechanisms, intermittent hypoxia and sleep fragmentation, which induce various intermediate disorders, such as activation of the sympathetic nervous system, oxidative stress, systemic inflammation, alterations in appetite-regulating hormones and activation of the hypothalamic–pituitary–adrenal axis, which in turn contribute to the development of insulin resistance, progression to glucose intolerance, and ultimately, to DM2 (Fig. 3).
In addition to continuous hypoxia,67–70 studies with animal models have shown that intermittent hypoxia can cause insulin resistance. In lean mice, exposure to successive intermittent hypoxia cycles reduced insulin sensitivity, compared to exposure to synthetic air, essentially due to reduced glucose utilization in oxidative muscle fibers.71 It appears that intermittent hypoxia only worsens glucose homeostasis during periods of hypoxic exposure, and induces pancreatic β-cell replication, probably to compensate for diminished insulin sensitivity.72 This compensatory response, however, is overridden by the presence of hyperglycemia, which increases apoptosis in β-cells and inhibits their replication.72
The effects of intermittent hypoxia on glucose metabolism in humans are less well known. However, Louis and Punjabi73 showed, in healthy volunteers subjected to intermittent hypoxia or normoxia, that the former was associated with a reduction in insulin sensitivity and glucose effectiveness.73 Moreover, intermittent hypoxia is accepted as one of the main determinants of HbA1c in patients with SAHS. A recent study evaluated factors related with HbA1c levels in 164 patients with SAHS and normal glucose tolerance, 111 with impaired tolerance, and 55 with diabetes.74 They found that HbA1c was correlated with minimum saturation in all patients, while no correlation was found with the AHI in patients with DM2 or normal blood glucose. This, therefore, suggests that the correlation between SAHS and HbA1c in patients with DM2 is more dependent on minimum SaO2 levels than on the AHI.74 Similarly, other authors report that the AHI is only associated with HbA1c during the REM sleep phase,75 which could in part explain individual variability. Another possible explanation for inter-subject variation lies in alterations in hypoxia-inducible factor-1, which triggers the expression of specific genes in the presence of a low oxygen levels.76 In diabetic rats, an increase in hypoxia-inducible factor-1 expression by pancreatic β-cells, which inhibits glucose transport and perpetuates a state of insulin resistance, has been reported.77
Sleep FragmentationIn laboratory conditions, complete sleep deprivation has been found to reduce glucose tolerance.46,78–80 Various observational studies in the general population have also shown a relationship between sleep deprivation and altered glucose metabolism. The Sleep Heart Health Study confirmed that less than 6h sleep is associated with a higher prevalence of diabetes or glucose intolerance than 7–8h of sleep.60 In addition, a considerable number of longitudinal studies indicate that sleep restriction increases the risk of developing diabetes. An analysis of the First National Health and Nutrition Examination Survey showed that fewer than 5hours sleep resulted in a 1.47-fold increase in the risk of developing diabetes during an 8–10 year follow-up.49 In more than 1000 men enrolled in the Massachusetts Male Aging Study, the risk of developing diabetes in subjects reporting a shorter average sleep time was double that of those sleeping for 7–8h.81 Although there is as yet little information on this aspect, the effect of sleep restriction on the risk of developing diabetes appears to be sex-dependent.82,83 In more than 70,000 non-diabetic adult women included in the Nurses Health study, sleeping less than 5h was found to increase the risk of developing diabetes in 10 years by 1.57-fold, although significance was reduced when adjusted for BMI and other confounding factors.47 Therefore, sleep restriction appears to be an independent risk factor for diabetes, primarily in men.
In addition to studies in the general population, several authors have evaluated the duration and quality of sleep in patients with diabetes.84,85 Most found that poor sleep quality was more prevalent in patients with diabetes,85 and that this negatively affected blood glucose control.84,85 The influence of quality of sleep on glucose tolerance has also been investigated in various longitudinal series.49,82,83,86,87 Apart from one study,83 all report an increased risk of diabetes associated with sleep disorders. The potential contribution of sleep fragmentation to the SAHS–diabetes relationship is evidenced by the importance of sleepiness, as demonstrated by Barceló et al.,88 who found that patients with SAHS and excessive daytime sleepiness have a higher HOMA index than non-sleepy patients with SAHS or healthy controls.88 The loss of REM sleep, due to sleep fragmentation, may also contribute to the development of diabetes.89 REM sleep has high energy requirements due to sustained neuronal activity, and is accompanied by an increase in cerebral glucose uptake as well as reduced insulin and glucagon levels.90 Therefore, the higher prevalence of diabetes in SAHS patients with a greater clustering of respiratory events during REM sleep91 could be related with fragmentation of this sleep phase.92
Activation of the Sympathetic Nervous SystemThe sympathetic nervous system plays an important role in the metabolic regulation of glucose and fat.93,94 Catecholamines are known to reduce insulin sensitivity, insulin-mediated glucose uptake,95 promote pancreatic β-cell apoptosis, and reduce insulin secretion.96,97 They can also inhibit insulin-mediated glycogenesis and increase glycolysis.95,96,98 Increased sympathetic activity has lipolytic effects, releasing circulating fatty acids, which reduce insulin sensitivity.93 Furthermore, sympathetic vasoconstriction may reduce glucose and insulin supply to skeletal muscles, directing the blood flow toward less metabolically active areas, which reduces the net glucose uptake.99
The succession of hypoxemia-reoxygenation periods that accompany apneas–hypopneas are known to affect the sensitivity of peripheral chemoreceptors in patients with SAHS,10,100 and stimulation of these receptors is known to increase sympathetic nervous system activity.10,101 Since activation of the sympathetic nervous system appears to have an impact on insulin sensitivity, it has been suggested that this plays a major role in the development of insulin resistance in patients with SAHS.102
Experimental evidence supports the suggestion that increased basal sympathetic tone plays an essential role in the SAHS-DM2 relationship. Glucose intolerance caused in healthy volunteers by exposure to periods of acute hypoxia is associated with an increase in plasma catecholamines.70 In healthy volunteers subjected to intermittent hypoxia, reduced insulin sensitivity and glucose effectiveness have been associated with a shift in the sympathovagal balance, predominantly toward sympathetic nervous system activity.73 However, blocking sympathetic activity in an animal model does not mitigate the short-term negative effects of intermittent hypoxia on insulin sensitivity, suggesting the involvement of other alternative pathways.71 In any event, this finding does not rule out that hypoxic activation of the sympathetic nervous system contributes to long-term progression of insulin resistance, which may occur for decades in patients with SAHS.
Oxidative StressAn excess of reactive oxygen species (ROS) may inhibit insulin-induced energy substrate uptake in muscle and adipose tissue, and may damage pancreatic β-cells due to their relatively low concentration of antioxidant enzymes.93,103 Moreover, the formation of ROS may suppress insulin secretion and worsen insulin sensitivity.104,105 In fact, studies at cellular level show that intermittent hypoxia has a negative effect on β-cell proliferation and death, which seems to be attributable to greater cellular oxidative stress.106
In animal models, intermittent hypoxia has been shown to stimulate the release of ROS, contributing to a proinflammatory state.107 As a result, plasma cholesterol levels increase, as hepatic uptake is inhibited,108 leading to insulin resistance, increasing insulin levels and reducing glucose tolerance.109 In healthy volunteers, intermittent hypoxia has also been shown to increase ROS,110 associated with decreased insulin sensitivity.73 Therefore, the increase in oxidative stress seen in patients with SAHS111,112 could contribute to the development of insulin resistance.
Systemic InflammationSeveral lines of evidence converge to implicate sub-clinical inflammation in the pathogenesis of insulin resistance and diabetes.93 Diabetic patients have a baseline proinflammatory state, characterized by high circulating levels of inflammatory cytokines such as interleukin-6, tumor necrosis factor-α, C-reactive protein and interleukin-18.113,114 Interleukin-6 and tumor necrosis factor-α in particular have been implicated in the pathogenesis of insulin resistance and DM2.115–117 Similarly, other studies have documented the existence of a proinflammatory state in patients with SAHS.84,118
Although the mechanism by which systemic inflammation contributes to the development of insulin resistance in SAHS is still not fully understood, macrophage recruitment and the lipotoxic pathway seem especially relevant. Chronic inflammation causes macrophage activation and accumulation in the pancreatic islets of patients with DM2, which could contribute to tissue destruction and abnormal repair.119,120 The lipotoxic effects of obesity may also play an important role in the pathogenesis of insulin resistance by activating the proinflammatory pathway. This is because adipocytes are a principal source of cytokines, which are secreted into the circulation depending on the size of the adipocyte, creating a directly proportional relationship between fat mass and circulating cytokines.121,122
Alteration in Appetite-regulating HormonesThe main hormones involved in this function are leptin, adiponectin and resistin.123 Leptin regulates hunger and weight gain at central level, increasing the hypothalamic expression of anorexigenic peptides and decreasing the expression of orexigenic peptides,124 while at peripheral level it appears to be implicated in glucose homeostasis.125 Adiponectin is synthesized by the adipocytes and regulates their sensitivity to insulin.126 Low circulating adiponectin levels are a major risk factor for diabetes, atherosclerosis and dyslipidemia.127 In contrast, high adiponectin concentrations have protective properties against diabetes.93 Although the role of resistin is more uncertain, it is thought to increase hepatic insulin resistance and reduce glucose tolerance.128
Some authors have reported that SAHS patients have lower plasma leptin levels than healthy subjects,80 although the role of obesity as a confounding factor has not always been properly controlled.129 This hormone appears to contribute significantly to the development of insulin resistance in SAHS. In leptin-deficient obese mice, intermittent hypoxia induces a decrease in blood glucose and an increase in serum insulin, both in the short and long term, consistent with greater insulin resistance.109 These responses are blocked when the animals are given a previous infusion of leptin,109 suggesting that the increased insulin resistance caused by the intermittent hypoxia is dependent on disruption of the leptin pathway.
Resistin is another adipokine that may contribute to obesity,130 insulin resistance131 and metabolic syndrome.132 Although some authors133 found no association between plasma resistin levels in patients with SAHS and insulin resistance, determined by the HOMA index, other studies show that the plasma resistin concentration is higher in SAHS patients with DM2 compared to those with glucose intolerance or normal glucose metabolism.134
Activation of the Hypothalamic–Pituitary–Adrenal AxisCortisol and other glucocorticosteroids interfere with carbohydrate metabolism by increasing glucose production, decreasing glucose uptake in peripheral tissues and inhibiting the release of insulin from the pancreatic β-cells.93,99
Activation of the sympathetic nervous system could increase the activity of the hypothalamic–pituitary–adrenal axis, increasing cortisol synthesis, which leads to insulin resistance and hyperglycemia.135 In fact, studies at altitude or under hypobaric conditions confirm that hypoxia modifies the function of the hypothalamic–pituitary–adrenal axis and increases circulating cortisol levels.135 Furthermore, alterations in the secretion of the appetite-regulating hormones may also affect glucose homeostasis, increasing nocturnal growth hormone levels and evening cortisol levels.46 There are few studies as yet in humans that have examined the changes in cortisol secretion induced by SAHS. Although one study indicated that SAHS does not affect cortisol levels,136 in other cases a cortisol concentration higher than that of weight-matched controls, which fell after CPAP treatment, has been described.137
In conclusion, based on the current information, we can establish a correlation between intermittent hypoxia and sleep fragmentation caused by SAHS and the development of insulin resistance, via various pathogenic pathways. Nevertheless, the molecular mechanisms of cell signaling that lead to increased insulin resistance in these patients are still not sufficiently understood.
Clinical and Prognostic Relevance of the Sleep Apnea–Hypopnea Syndrome–Diabetes AssociationIn recent years, a growing body of evidence has suggested that SAHS could increase the severity of DM2,138–140 but it also raises the interesting possibility of a reciprocal relationship.
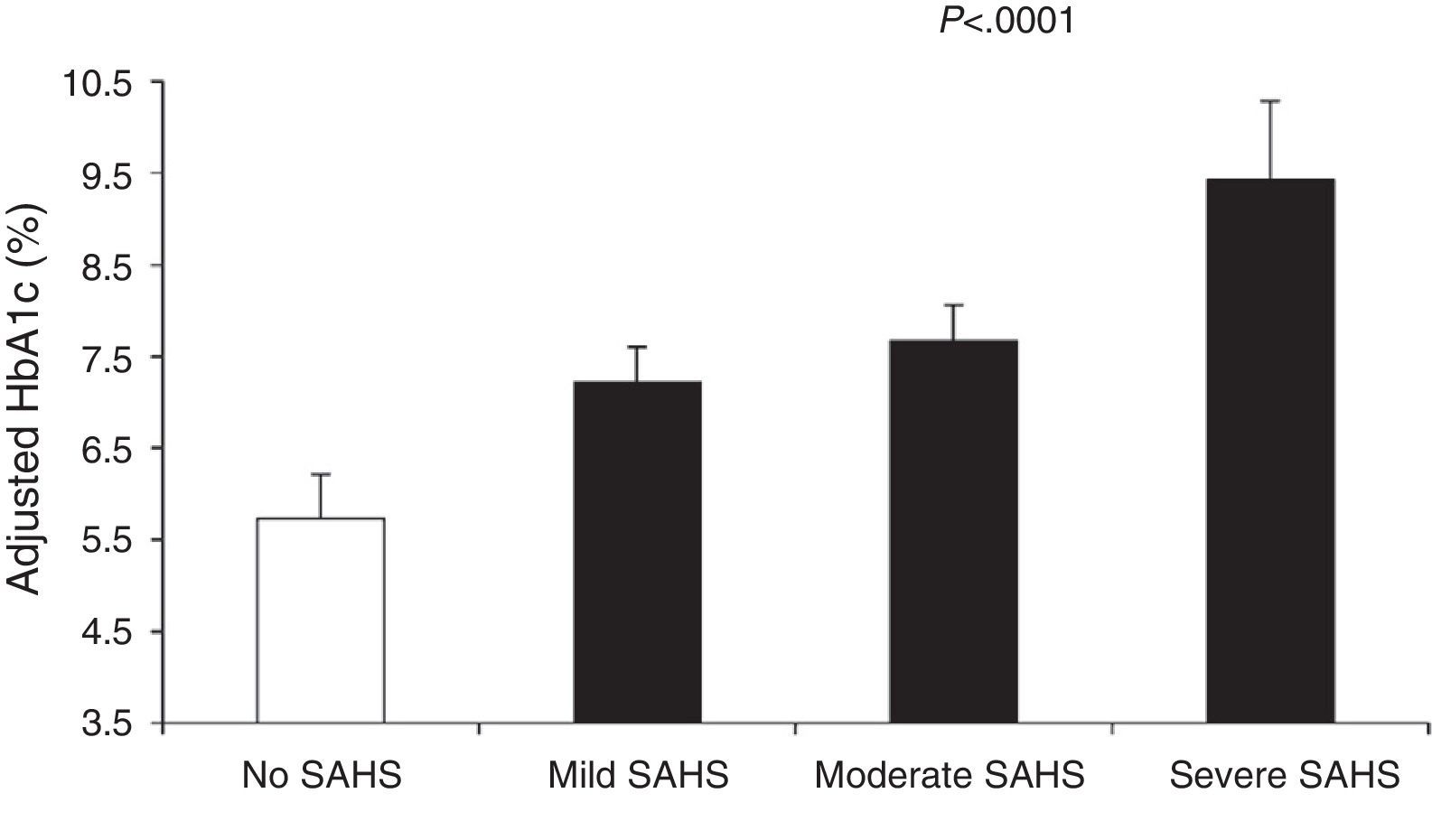
Aronsohn et al.141 showed that SAHS worsens blood glucose control in DM2. Compared to control subjects, mean adjusted HbA1c increased by 1.49% in patients with mild SAHS, 1.93% in moderate SAHS and 3.69% in severe SAHS (Fig. 4). Furthermore, other measures of SAHS severity, such as the AHI and desaturation during the REM phase, were also related to HbA1c levels.
Glycated hemoglobin (HbA1c) levels in controls and patients with varying SAHS severity. The values are adjusted for age, sex, race, body mass index, diabetes medication, level of exercise, years of diabetes and total sleep time. The bars represent the standard error of the mean. Reproduced with the permission of the American Thoracic Society. Copyright© 2014 American Thoracic Society. Aronsohn et al.141
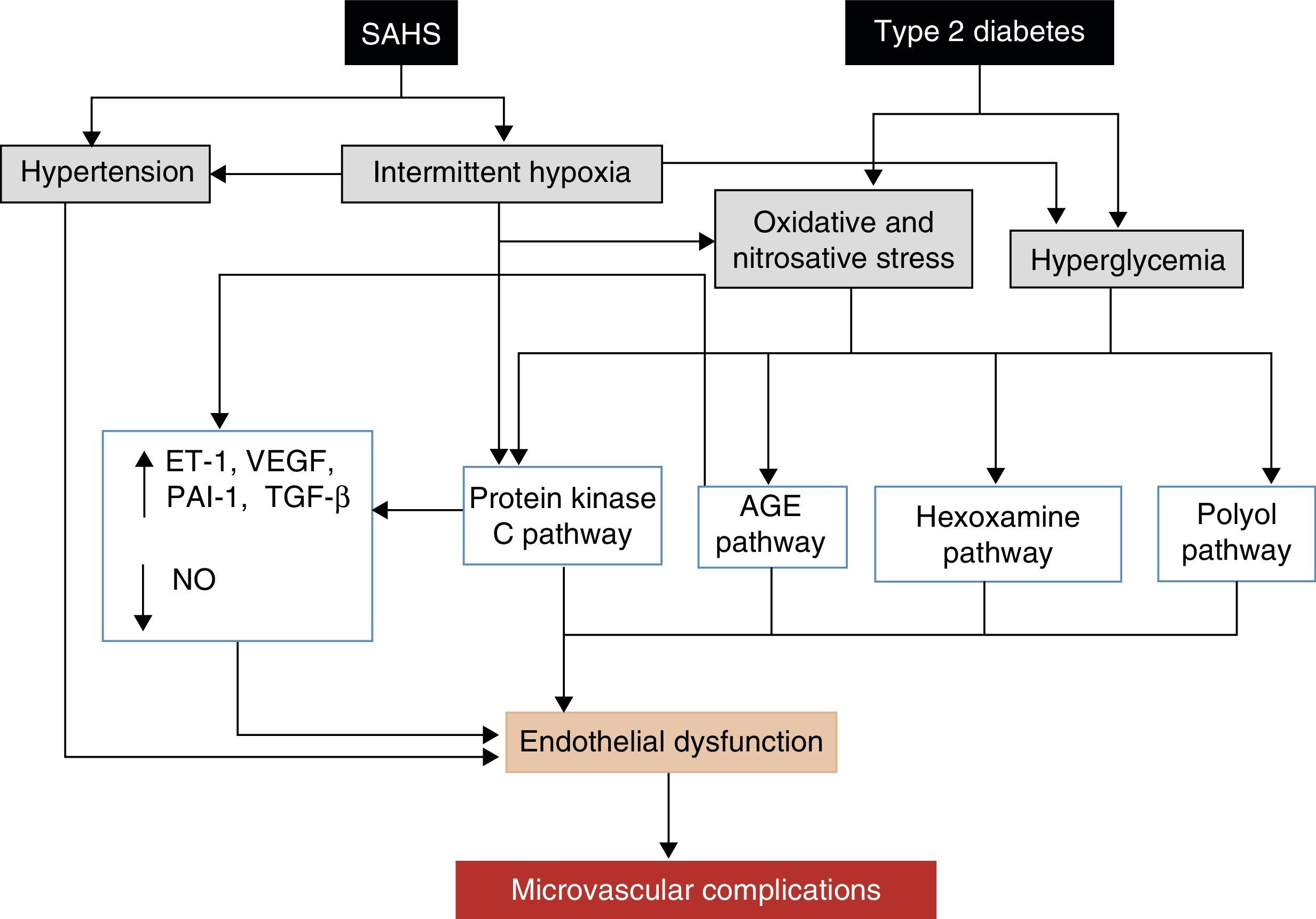
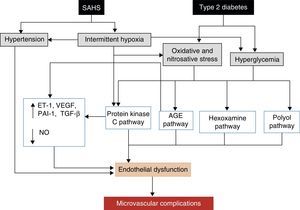
The leading cause of morbidity and mortality in patients with DM2 is the development of micro- or macrovascular complications, which can also be affected by SAHS. Macrovascular complications, which include ischemic heart disease, peripheral artery disease and cerebrovascular disease, are due to the harmful effects of hyperglycemia,142,143 as well as atherosclerosis. SAHS may also worsen certain typical microvascular complications, such as retinopathy, nephropathy and neuropathy.138,144 Various mechanisms are potentially involved in the development of microvascular complications.145 SAHS increases the production of advanced glycation end or waste products,146 and has been associated with an alteration in protein kinase C signaling, which plays an important role in the cell response to hypoxia.145 SAHS is also associated with a decrease in endothelial nitric oxide synthase and an increase in endothelin-1 levels,147 with hypercoagulability21 and inflammation.118 Repeated hypoxemia-reoxygenation episodes, meanwhile, induce ROS production.112 The final result of this process, aggravated by the effect of hyperglycemia and hypertension, is the development of endothelial dysfunction and microvascular impairment (Fig. 5).
Proposed mechanisms by which SAHS may contribute to the development of diabetic microvascular complications. AGE: advanced glycation end or waste products; ET-1: endothelin-1; NO: nitric oxide; PAI-1: plasminogen activator inhibitor-1; TGF-β: tissue growth factor-β; VEGF: vascular endothelial growth factor.
Despite some findings to the contrary,148 most studies show an association between SAHS and severity of retinopathy, as well as a higher risk of progression. In a cross-sectional case-control study that included 68 patients with non-proliferative diabetic retinopathy and 151 patients with proliferative diabetic retinopathy, the severity of nocturnal desaturations (evaluated by means of the desaturation index and the minimum SaO2) was a risk factor for proliferative diabetic retinopathy, suggesting that the reoxygenation caused by the SAHS could affect retinopathy progression.149 In another study conducted in men with DM2, SAHS was independently associated with retinopathy and diabetic maculopathy.139 A longitudinal study has also shown that patients with SAHS have a higher risk of developing advanced diabetic retinopathy.150 In a recent cross-sectional study in obese men with DM2, Rudrappa et al.151 described worsening of retinopathy but not of maculopathy, and a higher proportion of patients with proliferative diabetic retinopathy in the SAHS group. Moreover, SAHS was the only independent predictor of retinopathy after adjusting for new serum biomarkers of retinopathy and inflammation.151
Sleep Apnea–Hypopnea Syndrome and Diabetic NephropathyData on this other major complication of diabetes is contradictory. Schober et al.152 found a higher prevalence of nephropathy in adult diabetics with an AHI≥15 than in nonapneic patients. Although other authors have not identified a relationship between the presence of SAHS in patients with DM2 and the existence of microalbuminuria,140 in a group of patients with renal failure secondary to diabetes, SAHS was associated with a higher risk of hemodialysis and hypertension.153
In general, renal ischemia and hypoxia are considered to be key factors for the development of renal failure. Several studies suggest that an increase in the severity of SAHS is related with a deterioration in renal function, irrespective of established risk factors for nephropathy progression.144 Several mechanisms have been proposed to explain this. Intermittent hypoxia and sleep fragmentation caused by SAHS are known to activate the sympathetic nervous system and the renin–angiotensin–aldosterone system, increase cytokine levels, and contribute to oxidative stress. The increased generation of free radicals gives rise to a number of harmful processes, such as endothelial dysfunction, inflammation, platelet aggregation, atherosclerosis and fibrosis, which may increase the risk of renal damage in patients with SAHS. Furthermore, it is important to consider that SAHS is an independent risk factor of hypertension and glomerular hyperfiltration, and could be an independent predictor of proteinuria, a sign of renal disease and a risk factor for progression of chronic nephropathy to end-stage renal disease.144
Sleep Apnea–Hypopnea Syndrome and Diabetic NeuropathyDiabetic neuropathies are a heterogeneous group of disorders that affect different parts of the nervous system, including generalized symmetrical polyneuropathies, multifocal and focal neuropathies and autonomic neuropathy. Mayer et al.154 showed that patients with severe SAHS have peripheral nerve dysfunction caused by axonal lesions, the severity of which is partially related with nocturnal hypoxemia. Raman et al.155 later evaluated the risk of neuropathy in 1414 patients with DM2, and found that the group of diabetic women with altered sleep patterns had a higher risk of developing neuropathy. However, another study that only included 40 patients did not identify any association between AHI and diabetic neuropathy.156
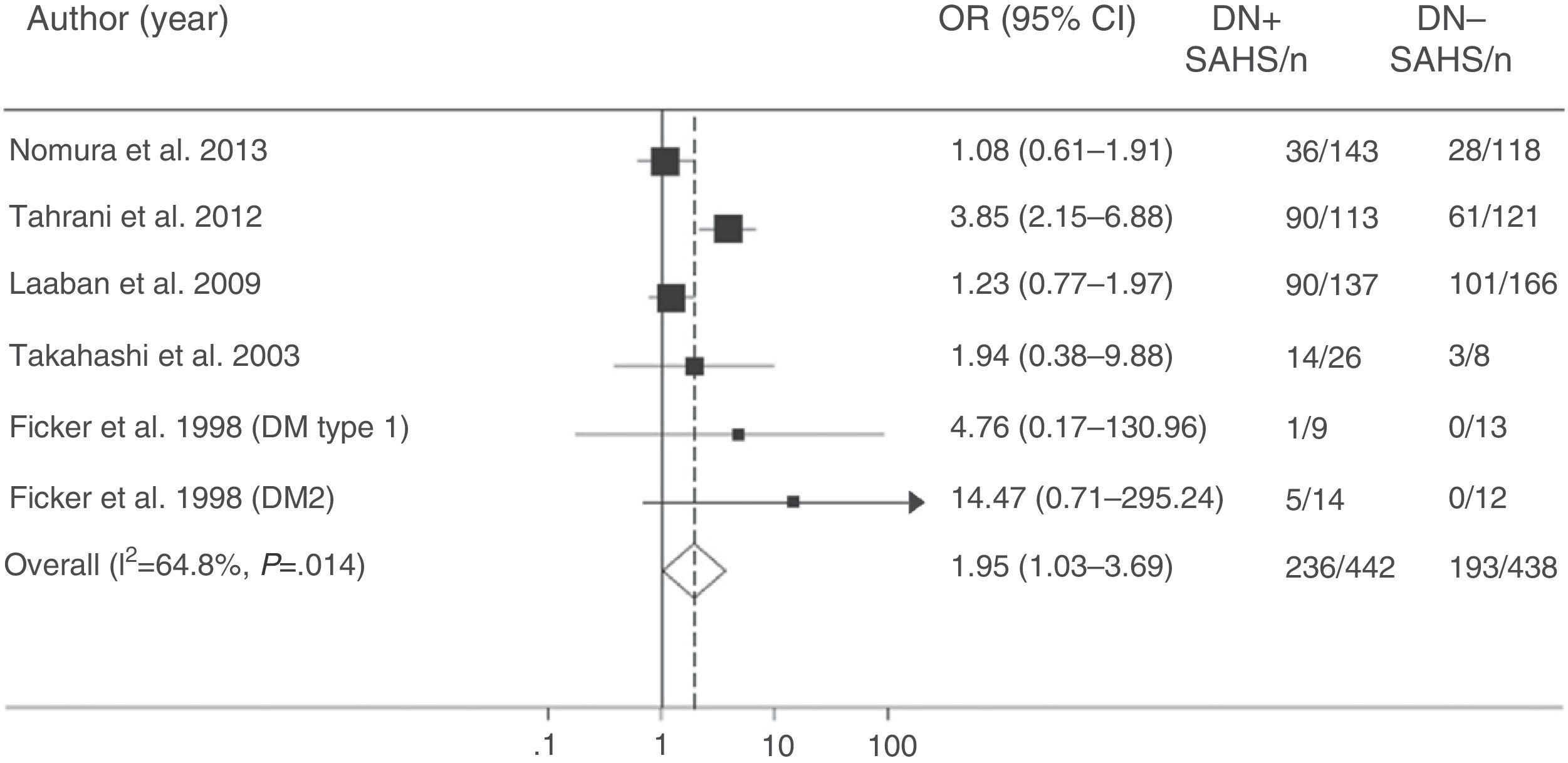
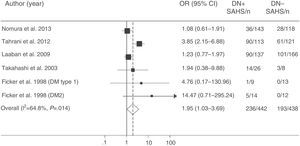
More recently, Tahrani et al.145 showed that SAHS is independently associated with diabetic neuropathy (adjusted odds ratio=2.82; 95% CI: 1.44–5.52). They found that the severity of diabetic neuropathy is related with the severity of both SAHS and nocturnal hypoxemia, and identified potential pathogenic mechanisms, including an increase in nitrosative stress.145 Finally, a meta-analysis confirmed the relationship between SAHS and diabetic neuropathy. The presence of SAHS was more frequent in DM2 patients with neuropathy (odds ratio=1.95; 95% CI: 1.03–3.70) (Fig. 6).157
Odds ratio (OR) with 95% confidence interval (95% CI) for the existence of SAHS in patients with diabetic neuropathy (DN) compared to subjects with no DN. The area of each black square is proportional to the statistical weight of each study. The white diamond represents pooled OR. The broken vertical line indicates the 95% CI of each OR. The solid vertical line represents the equivalence value between the 2 groups. Adapted from Fujihara et al.157
Although scant, some data suggest that DM2 might worsen the progression of SAHS (Fig. 3). In animal models, insulin resistance has been shown to be associated with a lower ventilatory response.158 Furthermore, the presence of diabetic neuropathy could increase upper airway collapsibility due to impairment of the dilatory muscles of the larynx, and aggravate obstructive apneas–hypopneas. Indeed, patients with autonomic neuropathy have more severe SAHS, with longer lasting respiratory events and more desaturation than diabetics with no autonomic neuropathy.159
Diabetes, moreover, may also contribute to the onset of central apneas. Diabetic patients in the Sleep Heart Health Study had a higher incidence of periodic breathing and central apneas than non-diabetic subjects.59 It has been suggested that diabetes-associated autonomic neuropathy alters upper airway reflex responses, peripheral control of upper airway muscles, mechanoreceptor activation thresholds, central ventilatory stability and control, and peripheral response to hypercapnia and hypoxia.93,160 Chronic hyperglycemia, moreover, induces oxidative stress, causing nerve dysfunction and structural damage,161 which could worsen autonomic dysfunction and aggravate sleep disordered breathing, creating a vicious circle. It is precisely this relationship that explains the effect of some general interventions that are beneficial in SAHS. Exercise and increased physical activity, therefore, which can improve blood glucose control, could also have a beneficial effect on the severity of SAHS that cannot be fully explained by the associated weight loss.93,162 Moreover, some studies show that the weight loss is associated with substantial improvement in the severity of SAHS due to changes in the structure and function of the upper airways.163,164
In conclusion, there is growing evidence to associate SAHS with the development of DM2. Further evidence of this is the existence of common pathogenic pathways that could have important prognostic implications, not only for the progression of diabetic complications, but also for a synergic effect on SAHS itself.
FundingPartially funded by the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) (034/2013), the Health Research Fund (PI10/00642 and PI13/01512), the Ministry of Science and Education (SAF2007-62270) and the Program for R&D in Biomedicine in the Madrid Region (S2010/BMD-2542).
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: Martínez Cerón E, Casitas Mateos R, García-Río F. Síndrome de apneas-hipopneas del sueño y diabetes tipo 2. ¿Una relación de ida y vuelta? Arch Bronconeumol. 2015;51:128–139.