Animal models of disease have always been welcomed by the scientific community because they provide an approach to the investigation of certain aspects of the disease in question.
Animal models of COPD cannot reproduce the heterogeneity of the disease and usually only manage to represent the disease in its milder stages. Moreover, airflow obstruction, the variable that determines patient diagnosis, not always taken into account in the models. For this reason, models have focused on the development of emphysema, easily detectable by lung morphometry, and have disregarded other components of the disease, such as airway injury or associated vascular changes.
Continuous, long-term exposure to cigarette smoke is considered the main risk factor for this disease, justifying the fact that the cigarette smoke exposure model is the most widely used. Some variations on this basic model, related to exposure time, the association of other inducers or inhibitors, exacerbations or the use of transgenic animals to facilitate the identification of pathogenic pathways have been developed. Some variations or heterogeneity of this disease, then, can be reproduced and models can be designed for resolving researchers’ questions on disease identification or treatment responses.
El desarrollo de modelos animales de una enfermedad ha sido siempre bien acogido por la comunidad científica porque permite realizar una aproximación a la investigación de determinados aspectos de la misma.
Los modelos animales de la EPOC no pueden llegar a reproducir la heterogeneidad de esta enfermedad y generalmente solo llegan a representar los estadios más leves de la misma. Además, la obstrucción al flujo aéreo, variable que determina el diagnóstico en un paciente, no siempre se tiene en cuenta en los modelos. Por este motivo, los modelos se han centrado en el desarrollo de enfisema, fácilmente detectable por morfometría pulmonar, sin prestar atención a otros componentes de la enfermedad, como la lesión de las vías aéreas o las alteraciones vasculares asociadas.
La exposición continua y prolongada al humo de tabaco se considera el principal factor de riesgo de esta enfermedad, lo que justifica que sea el modelo de exposición al humo de tabaco el más ampliamente utilizado. Sobre esta base de modelo podemos encontrar algunas variantes relacionadas con el tiempo de exposición, la asociación de otros inductores o inhibidores, las exacerbaciones o el uso de animales transgénicos que facilitan la identificación de las vías patogénicas. Es posible, por tanto, reproducir algunas variantes o heterogeneidades de esta enfermedad y diseñar uno u otro modelo que sea capaz de responder a una u otra pregunta de investigación, dirigida bien a una identificación patogénica y/o bien a una respuesta terapéutica.
Chronic obstructive pulmonary disease (COPD) has a huge global impact, and clinicians must make use of all the tools available to tackle the many aspects of this disease. The development of animal models can help address problems such as under-diagnosis, frail exacerbator patients, and existing uncertainties about the development of one or other clinical form of the disease or its natural history (in which cases with minor and accelerated disease progression are mixed). Similarly, all new therapeutic trials are generally based on an earlier study in an animal model.
Smoking is the leading cause of COPD, but its ability to generate a permanent inflammatory response depends on the patient's susceptibility. For this reason, animal models of COPD developed by exposure to cigarette smoke are primarily chosen to study the pathogenic mechanisms of the disease and of susceptibility to development and progression. In these cases, the use of transgenic animals, in which a particular metabolic pathway is inhibited or activated, helps researchers understand the pathogenic pathways that exist in each case. Likewise, new approaches to the classification of COPD proposed by both the GOLD initiative1 and Spanish COPD guidelines (GesEPOC)2 place greater emphasis on exacerbations due to their effect on the severity of symptoms, progression of the obstruction, and mortality. For this reason, interest has grown in the study of exacerbations in models of COPD, and the results may help to improve knowledge of the mechanisms underlying the condition in the exacerbator patient.
When evaluating the results of studies in animal models of any disease, the limitation of having to extrapolate a conclusion as to what is potentially present in a patient must always be taken into consideration. However, they are an essential part of clinical research when used as “preclinical models”, an increasingly widespread term that encompasses the notion of translation into clinical practice that must form the basis of any study design.
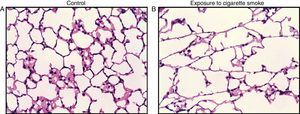
Models of Cigarette Smoke-Induced COPDModels of cigarette smoke-induced COPD are those that best reflect the inflammatory and pathogenic mechanisms of the disease and, consequently, those that are potentially better suited to testing new therapies. Exposure to cigarette smoke has been applied in numerous animal species, such as dogs, guinea pigs, rabbits, rats, and mice. Of these, guinea pigs and mice have proven to be most susceptible to the development of COPD through prolonged exposure.3 There are two general procedures for administering cigarette smoke: the so-called “nose only” method, where the smoke is channeled directly into the animal's nose, and “whole body” administration (Fig. 1), where the animal is placed in a chamber filled with a controlled concentration of smoke to ensure complete exposure to stable, non-toxic carboxyhemoglobin levels.4,5 Conceptually different, both methods have been widely used, and have shown similar findings as regards the presence of inflammatory cell populations, cytokine levels, changes in lung remodeling, and therapeutic response6 (Fig. 2).
In approximately 90% of patients, COPD is caused by smoking an average of at least 10 pack-years, and they develop a disease that can take different clinical forms, with different levels of progression and severity.7 Animal models of COPD, guinea pig or murine are usually established over a 6-month exposure period,8 although major inflammatory and morphometric changes can already be detected after the second month.9 They do not usually reach the stage equivalent to severe COPD in a patient, but they can develop many of the characteristics typical of this disease, such as chronic inflammation with increased neutrophil and macrophage counts, presence of CD4 and CD8T lymphocytes, mucus hypersecretion, changes in lung function, emphysema, and vascular and airway remodeling.10
The murine model of cigarette smoke exposure is the most widely used, due to its low cost and easy management, well-mapped genome, the availability of many transgenic variants, a wide range of specific antibodies for laboratory use, and a large number of strains with differing susceptibilities to cigarette smoke. Strain-dependent susceptibility for developing COPD is well identified in the murine model,11–14 and the pulmonary morphometric pattern of COPD can be generated when mice are exposed to cigarette smoke for at least 3 to 6 months, with typical inflammatory cells, inflammatory mediators and functional changes characteristic of the disease.15
Guinea pigs are also a good choice for generating models of COPD, as these animals are very susceptible to developing the disease after only a few months of exposure.16 In 1990, Wright and Churg published one of the first studies in guinea pigs exposed to cigarette smoke.17 In this case, after 12 months of exposure the guinea pigs developed emphysema and presented lung function changes very similar to those found in smokers with COPD. The main difficulty in the use of this species comes from the limited availability of specific antibodies. Rats, the species of rodent closest to mice and guinea pigs, are rarely used as models of COPD because they are more resistant to developing changes due to cigarette smoke exposure.18Table 1 summarizes some relevant findings described in this model.
Examples of Cigarette Smoke-Induced COPD.
| Animal model | Methodology | Finding | Reference |
|---|---|---|---|
| Serpin B1 KO mice | Chronic exposure | Not associated with severe emphysema | 83 |
| NZWLac/J, AJ, SJL, C57BL/6, and AKR mice | Chronic exposure | The degree of susceptibility to developing lung injury is strain-dependent | 12 |
| NZWLac/J and AKR mice | Chronic exposure | Egr-1 (proinflammatory marker) greatly increased in the susceptible AKR strain, but far less so in the NZWLac/J strain | 84 |
| C57BL/6 (CD8−/−) mice | Chronic exposure | CD8+ T lymphocytes are essential for the development of emphysema | 85 |
| C57BL/6 mice | Acute exposure | Increased markers of DNA damage by oxidative stress (8-OHdG and 4-HNE) | 86 |
| ICR (Nrf2−/−) mice | Chronic exposure | More susceptible to developing emphysema, with increased antioxidant enzyme expression | 78 |
| Guinea pig | Chronic exposure | Increase in vascular remodeling and arterial pulmonary pressure | 87 |
| C57BL/6 and “pallid” mice | Chronic exposure | The α1-antitrypsin levels determine the emphysematous profile | 88 |
| Guinea pig | Chronic exposure | Role of MMPs in the development of emphysema and airway remodeling | 89 |
| C57BL/6 mice | Acute exposure (direct and indirect) | The intensity of the inflammatory response depends on the composition of the cigarette smoke | 90 |
| C57BL/6 mice (sGCα1(−/−)) | Acute and chronic exposure | Reduction in sGC expression contributing to airflow limitation | 91 |
| Mice | Sub-chronic exposure | Anti-inflammatory effect of PPAR-γ ligands | 92 |
| Mice | Chronic exposure | The neutralization of CXCL13 partially blocks the inflammatory response and alveolar wall destruction | 93 |
| Mice | Chronic exposure | Blocking T lymphocytes may be effective as therapy for COPD | 94 |
| C57BL/6 and BALB/cJ mice | Chronic exposure | Cigarette smoke triggers an antigen-dependent response in which CD4+ and CD8+ lymphocytes participate | 95 |
| C57BL/6 and 129S2/SvHsd mice | Acute exposure | Implication of the pro-inflammatory monocytes in susceptibility to developing lung injury | 5 |
Models of COPD due to chronic cigarette smoke exposure continue to be limited insofar as they are unable to reproduce some of the characteristics of this complex, heterogeneous disease. So far, attempts to develop known clinical phenotypes, such as the exacerbator or accelerated progression types, or forms with bacterial colonization, for example, have failed. Some studies, however, have attempted to address these questions by combining agents. Exposure to toxic and irritant gases such as nitrogen dioxide, ozone or sulfur dioxide causes more severe lung damage than cigarette smoke.19–22
Models of COPD ExacerbationsExacerbations are a characteristic of COPD that, if repeated, determine the poor clinical course of the patient, as they are associated with greater disease progression, poorer quality of life and higher risk of mortality. The availability of animal models of exacerbation gives researchers the chance to study associated pathogenic mechanisms and detect possible associated biological markers.
Most infectious COPD exacerbations are viral in origin (75%), while the remainder are bacterial. Studies in in vivo models have demonstrated the effect of viral infection on mice previously exposed, both short- and long-term, to cigarette smoke. Inflammation of the lung is more severe if the viral infection affects an animal previously exposed to cigarette smoke, and also accelerates emphysema progression and the severity of airway damage.23,24
The most commonly isolated bacterium in COPD exacerbations is nontypeable Haemophilus influenzae (NTHI). For this reason, the results obtained in models of this infection in healthy rats25 vs. those previously exposed to cigarette smoke are particularly interesting. After C57BL/6 mice had been exposed to cigarette smoke for 8 weeks, NTHI infection caused a more severe inflammatory response and greater lung damage than in previously healthy animals.26,27
Bacterial lipopolysaccharides (LPS) have been used alone, in long-term administration,28 or in combination with short periods of exposure to cigarette smoke29 to develop models of emphysema. However, single massive insult can cause an inflammatory response that is accompanied by fever, mucus hypersecretion and bronchoconstriction, which reproduces symptoms of an exacerbation30 seen on computed tomography.31
Models of Severe COPD by Combining Induction AgentsIn the more severe stages of COPD a clear breakdown of the lung “maintenance program” occurs that can inevitably lead to emphysema and pulmonary hypertension. There are various models of “frail” (very severe) COPD pathology, such as the combination of cigarette smoke exposure and vascular endothelial growth factor (VEGF) inhibitor.32 Exposure to cigarette smoke causes a significant decrease in VEGF and VEGF receptor-2 (VEGFR-2) expression in animal models of emphysema. Furthermore, treatment with the VEGF receptor blocker SU5416 induces alveolar cell apoptosis, capillary retraction, and alveolar space enlargement.33 For this reason, emphysema presents as a VEGF deficiency that compromises the survival of the endothelial cells and consequently the lung's maintenance program. Other results can be achieved by combining cigarette smoke exposure and hypoxia induction. This model can lead to pulmonary hypertension, a condition present only in advanced severe COPD.34
Transgenic Models of COPDBefore the advent of targeted genetic engineering, some mutant strains of C57BL/6 mice that spontaneously developed emphysema had appeared (Table 2). These were the “blotchy” mice that have an abnormal translation of the Menkes gene on the X chromosome,35 causing defects in lung connective tissue proteins, which affects the structure and function of the lungs, causing emphysema36; “Tight Skin” mice, with a mutation in fibrillin-1, one of the key components of the microfibrils in the lung extracellular matrix, which causes oxidative stress and cell death, injury cascades central to the development of emphysema37,38; “Beige” mice, in which the lungs appear normal at birth but, due to the deletion of Lyst, do not form alveoli normally during development39; “Pallid” mice,40 with a mutation that affects syntaxin-13 (a cell membrane protein), resulting in the gradual and progressive development of emphysema41; and more recently, “Osteopetrotic” mice, which are macrophage colony-stimulating factor-deficient and eventually develop emphysema.42 One of the major technological breakthroughs of the last few decades has been the development of transgenic animals. These are animals in which a gene that does not form part of their genome, and which will sequence a certain pathway of interest, is inserted by intranuclear injection in the early embryonic phases.13,15,43–45 One of the first applications of transgenic technology to COPD was the constitutive overexpression of human collagenase-1 (MMP-1) in mice, which causes emphysema46 by degradation of type III collagen in the alveolar walls.47 The constitutive expression of transgenes, however, does not distinguish the lung's own development process. To overcome this, the transgenic expression construct was developed. Thus, overexpression of IL-13,48 a cytokine produced by T-helper type 2 (Th2) lymphocytes, or overexpression of IFN-γ,49 the main product of T-helper type 1 (Th1) lymphocytes, are two important examples of inducible conditional transgenes. In the case of IL-13 transgenic mice (“Dutch”), this leads to MMP-9 and MMP-12-dependent emphysema in adult mice.50 In these animals, IL-13 is overexpressed only when they are exposed to tetracycline, thereby allowing investigators to activate overexpression after the lung is fully developed. MMP-9-mediated activation of TGF-β appears to be responsible for collagen remodeling in this model. However, in IFN-γ transgenic mice (“British”), the inflammatory component appears to be more subtle, with prominent apoptosis but no associated airways disease. These are only two examples that demonstrate the complexity of inflammatory networks, and how unexpected findings in animal models have led to the search for new mediators in human disease. Other studies show how TNF-α induction in the adult lung facilitates the formation of lymphoid tissue and emphysema, providing a model for research into the pathogenic effects of TNF-α in the lung,51 or how prothymosin-α (ProT-α) expression contributes to the pathogenesis of emphysema by increasing acetylation of histones and expression of NF-κB-dependent MMP-2 and MMP-9, especially after cigarette smoke exposure.52
Strains of Natural Mutant Mice That Develop Emphysema.
| Mouse | Mutation | Pulmonary phenotype | Reference |
|---|---|---|---|
| Blotchy (Blo) | Abnormal translation in the Menkes gene on the X chromosome | Air space enlargement | 35,36 |
| Disorganization of the elastic fibers | 37,38 | ||
| Tight skin (Tsk+/−) | Duplication of fibrillin-1 | Abnormal air space development, with development of panlobular emphysema | 39 |
| Beige (Bg) | Deletion in Lyst | Normal at birth, but with abnormal neonatal alveolization | 40,41 |
| Pallid (Pa) | Syntaxin-3 | Develop mild, late onset emphysema | 42 |
| Osteopetrotic (Op) | Macrophage colony-stimulating factor deficiency | Reduced number of alveolar macrophages and emphysema | |
An alternative to the transgenic model is the “knockout” model (Table 3), in which the expression of a certain gene is inhibited, thereby enabling the function of proteins dependent on this gene to be determined. Sometimes, gene inhibition protects against the development of emphysema, as in the case of inhibition of MMP-12 expression, which impairs alveolar macrophage recruitment and thereby protects against the development of emphysema.53 Another example is the absence of neutrophil elastase (NE), which also appears to protect against the development of smoke-induced emphysema.54 In both cases, the direct role of these proteins in emphysema has been demonstrated, highlighting the interdependence of the proteinases and inflammatory cells that mediate lung destruction in response to cigarette smoke. At other times, the deletion interferes with alveogenesis. In this case, platelet-derived growth factor A (PDGF-A)-deficient mice develop emphysema due to loss of myofibroblasts and the associated elastin fiber deposits.55 Double knockout mice for fibroblast growth factor receptors 3 and 4 (FGFR-3 and -4) have abnormal alveolar formation and septation,56 while elastin-deficient mice have fewer dilated distal air sacs and arrested airway development.57 In other cases, deletion of certain genes causes alveolar space enlargement. This is true of integrin αVβ6-deficient mice, in which TGF-β activation in alveolar air spaces does not occur, leading to development of MMP-12-dependent emphysema.58 Other examples are knockout mice for pulmonary surfactant protein D (SP-D), which present macrophage activation, production of MMPs and air space enlargement,59 or tissue inhibitor of metalloproteinase-3 (TIMP-3) deficiency, which appears to combine air space enlargement with the gradual development of emphysema.60
Examples of “Knockout” Models of Emphysematous Mice.
| Gene | Pulmonary phenotype | Reference |
|---|---|---|
| Macrophage elastase (MMP-12) | Complete protection against the development of emphysema after exposure to cigarette smoke | 53 |
| Neutrophil elastase (NE) | Protection against cigarette smoke-induced emphysema | 54 |
| Elastin | Fewer dilated distal air sacs and arrested airway development | 57 |
| Platelet-derived growth factor A (PDGF-A) | Lack of tropoelastin and failure in alveolar septation | 55 |
| Fibroblast growth factor receptor (FGFR-3 and -4) | Abnormal alveolar formation and septation | 56 |
| Integrin αvβ6 | Spontaneous development of MMP-12-dependent emphysema | 58 |
| Pulmonary surfactant protein D (SP-D) | Macrophage activation, production of MMPs and subsequent emphysema | 59 |
| Tissue inhibitor of metalloprotease-3 (TIMP-3) | Air space enlargement with gradual development of emphysema | 60 |
To overcome the cross-species barrier in the murine model, certain murine genes can be eliminated and human genes inserted (“knocked in”) under the control of murine promoters. Emphysema-prone mice, in which the murine alpha-1 antitrypsin (A1AT) genes have been removed and replaced by normal or deficient human A1AT genes, have also been developed.61
Autoimmune Models of COPDPulmonary inflammation in severe COPD involves a large number of activated Th1T lymphocytes, B lymphocytes and CD8 lymphocytes, which persist for years, even after smoking cessation; this is consistent with a self-perpetuating process, which is one of the characteristics of autoimmune diseases. This chain of events suggests that the adaptive immune response in COPD, together with its persistence after smoking cessation, could be due to a response to autoantigens. Initially, this was merely a hypothesis,62–64 but since then new evidence, including the development of the first animal model of autoimmune emphysema,66–68 would seem to confirm the suggestion.65 The presence of anti-elastin autoantibodies69 and other autoantigens70,71 has been correlated with emphysema severity, and induction of autoantibodies against lung matrix proteins has been shown to increase the smoke-induced immune response in mice previously immunized with a mixture of lung extracellular matrix proteins.72
Models for Therapeutic Trials in COPDCurrent treatments do little to inhibit chronic inflammation, do not reverse COPD pathology, and do not modify the factors that initiate and lead to disease progression in the long term. It is clear, therefore, that new therapies that can prevent COPD induction and progression must be developed, and this is only possible through animal models that accurately reflect the physiopathology of the disease. Many anti-COPD drugs in clinical development have been identified from studies in animal models. Various inhibitors of inflammatory mediators are being developed for the treatment of COPD, although to date the results of tests using LTB4, TNF-α, IL-1, IL-8, and EGF inhibitors have been disappointing.73 Studies in animals exposed to cigarette smoke and treated with synthetic neutrophil elastase inhibitors have shown their potential anti-inflammatory activity.74 Similarly, findings in animal models of cigarette smoke-induced airway inflammation support the potential therapeutic usefulness of kinase inhibitors (p38 MAPK and PI3K) in COPD.75 The antioxidant enzyme Gpx-1 protects against lung inflammation and cigarette smoke-induced emphysema in mice,76 and a Gpx mimetic also reduced lung inflammation when administered both prophylactically and therapeutically.76,77 In other studies, deletion of the Nrf2 antioxidant stress response gene led to increased lung inflammation and emphysema in mice exposed to cigarette smoke,78 and an Nrf2 activator is currently undergoing clinical trials for COPD.73
New COPD drugs that can reduce the rate of pulmonary destruction and airflow limitation, and even arrest or reverse the underlying processes have yet to be discovered. In this regard, some evidence suggests that retinoic acid significantly slows elastase-induced emphysema in rats,79 and this has sparked interest in the retinoids80 and other growth factors as potential therapeutic agents,81 although how these can be used has yet to be confirmed. Studies published by our group show that liver growth factor (LGF) has pulmonary anti-fibrotic activity, and can improve lung function and partially revert the deposit of matrix proteins following CdCl2 administration in a model of fibrosis in rats.82
In conclusion, studies in animal models of COPD continue to contribute important information. These valuable tools further our understanding of the pathogenic aspects of the disease and help develop therapeutic clinical trials. The inherent heterogeneity of the disease can also be reflected in animal models developed by using different combinations or doses of induction agents. This is why it is important to choose the model according to whether the research is focused on pathogenesis, diagnosis or treatment.
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: Pérez-Rial S, Girón-Martínez Á, Peces-Barba G. Modelos animales de enfermedad pulmonar obstructiva crónica. Arch Bronconeumol. 2015;51:121–127.