We report the case of a 6-month-old boy with severe pulmonary hypertension (PHT) who presented clinical worsening with acute pulmonary edema (APE) after starting conventional vasodilator treatment. He was referred from another center for a second opinion and to complete the PHT study. Parental consent was obtained for the publication of this case report and images.
The patient was monitored during gestation, and had negative serologies and normal ultrasound scans. He was delivered at term with an appropriate weight for his gestational age and no perinatal incidents. Family history was significant for a spontaneous abortion and 2 healthy living siblings from the mother. At 3 months of age, he began to develop difficulty with ingestion, growth impairment, and hypotonia with mild psychomotor retardation.
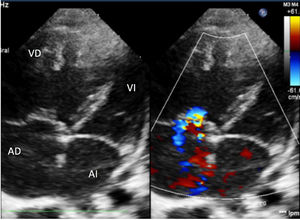
He was admitted to the ward and received oxygen therapy, oral sildenafil, and inhaled prostacyclins. After 5 days, his situation suddenly worsened with desaturation and low cardiac output, so he was transferred to the pediatric ICU. Mechanical ventilation began with a FiO2 of 1, nitric oxide up to 20 ppm, and inotropic support with dopamine. X-ray revealed Kerley B-lines and mottled perihilar infiltrates, while thoracic ultrasound showed homogeneous increase in B-lines, with no condensations or pleural effusion. Echocardiography showed signs of severe PHT (Fig. 1) with an estimated pulmonary artery pressure (PAP) of 70 mmHg (normal value [NV] < 20 mmHg). Cardiac catheterization was performed which showed: systolic PAP/diastolic (mean) PAP of 53/24 (38) mmHg (NV systolic PAP < 35 mmHg, mean PAP < 20 mmHg),1 pulmonary resistances of 8.51 Wood units/m2 (NV < 3 WU/m2), and pulmonary artery wedge pressure (PWP) of 10 mmHg (NV < 15 mmHg).1 Left heart changes were thus excluded as the cause of PHT.
Echocardiography. 4-chamber view. Dilated right cavities with interventricular septum deviated to the left. Moderate tricuspid regurgitation with a gradient of 60 mmHg allowing estimation of a PAP of approximately 70 mmHg. Interatrial septum with 5 mm patent foramen ovale. Valves of normal morphology.
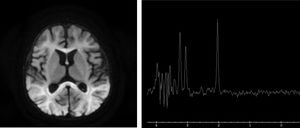
The metabolic study was expanded, showing hyperlactacidemia with 8.1 mmol/L lactate (NV < 2 mmol/L) and no metabolic acidosis or ketosis, increased blood glycine of 1,599 µmol/L (NV 109-293 μmol/L), and 83.1 μmol cerebrospinal fluid (CSF) (NV 3-13 μmol). Non-ketotic hyperglycinemia (NKHG) was suspected, and treatment began with thiamine, sodium benzoate, lipoic acid and acetyl cysteine, and brain magnetic resonance imaging (MRI) was performed (Fig. 2), revealing reduced subcortical deep white matter, while altered myelination with glycine peak in the right parietal white substance was observed on spectroscopy (Fig. 2).
Despite all efforts, the patient’s severe PHT persisted and he died 28 days after admission. The result of the genetic study was received 2 weeks later, confirming mutation in the NFU1 gene (c.565 G > A, p.Gly189Arg), described in the literature as pathogenic, causing mitochondrial dysfunction in multiple organs.
PHT is rare in pediatrics and mainly associated with inborn errors of metabolism. Prognosis in these cases is fatal.1,2 In all infants with PHT, any association with metabolic disease should be ruled out, as PHT may appear before neurological manifestations.3 Specifically, if the patient develops associated neurological manifestations, signs of multiorgan failure, or if APE occurs with conventional treatment,2–7 a metabolic study should be performed to check for an increase in glycine levels in plasma, CSF and urine, as was performed in our patient.2,4–6 The combined defect of pyruvate dehydrogenase complex activity and different respiratory chain complexes in skeletal muscle, skin fibroblasts and liver has also been defined.2,4–6
So far, 3 phenotypes associated with either neurological or respiratory symptoms or both have been described.2 In our case, the patient had PHT with hypotonia and slightly delayed psychomotor development, so he could be included in the third group. As in the cases described in the literature, leukoencephalopathy was observed on MRI and glycine peak was seen in the right parietal lobe.2,4–6
In the series described so far, the onset of PHT occurs in the first 6 months of life, it does not respond to usual treatment, and the prognosis is unfavorable, with the patient dying in the first 15 months of life.2,4 In addition, clinical worsening after initiation of vasodilator treatment is described in all cases.
Invernizzi et al. and Nizon et al. published the case of 2 patients who survived longer, dying at 30 months of age.5,6 Both cases were associated with the same allelic alteration of the NFU1 gene (c.565G > A, p.Gly189Arg). The other patients had different mutations of the NFU1 gene and died earlier.
Our patient’s genetic study revealed 2 mutations in the NFU1 gene. One had been previously described as pathogenic (P.Gly208Cys/c.622G > T),2,8–11 but the second sequenced mutation (P.Trp130Arg/c. 388T > C) was classified as a variant of uncertain meaning. Since this mutation has not been previously described in a disease with recessive inheritance, it should be considered in future research in case it is a pathogenic mutation. NFU1 gene mutations cause mitochondrial dysfunction in multiple organs with an autosomal recessive pattern of inheritance.
NFU1 gene alterations decrease or eliminate the production of the protein involved in the formation of iron sulfide complexes that bind to certain proteins and are required for their proper function, such as the production of mitochondrial energy and the decomposition of glycine. This phenomenon explains the laboratory and clinical findings of our patient.2
In conclusion, PHT associated with inborn errors of metabolism is rare and confers a bleak prognosis. It should be suspected in an infant with severe PHT and sometimes neurological symptoms, who presents clinical worsening with PAE after initiation of conventional treatment, and hyperlactacidemia without metabolic acidosis. Therefore, in all cases of idiopathic PHT, metabolic studies should be performed. It is important to reach a diagnosis in order to offer of genetic counseling because this disease is fatal and no curative treatment has been described to date.
Please cite this article as: Estepa Pedregosa L, Guitart Pardellans C, Baucells Lokyer BJ, Prada Martínez FH, García Cazorla A, Cambra Lasaosa FJ, et al. Hipertensión pulmonar grave como inicio de la enfermedad metabólica. Arch Bronconeumol. 2020. https://doi.org/10.1016/j.arbres.2020.06.020