Pathogenesis: The most frequent mechanism in nosocomial pneumonia is the aspiration of microorganisms that colonize the oropharynx and/or the gastrointestinal tract.
The origin of the causal agents of the colonization and infection can be exogenous, when the pathogen comes from the environment, or endogenous, when it comes from the bacterial flora of the patient.
In patients with endotracheal tubes, the formation of the bacterial biofilm is an important source of infection in ventilator-associated pneumonia (VAP).
Risk factors: These are divided into two groups: (a) clinical situations that alter the defense mechanisms of the host (intrinsic); and (b) diagnostic–therapeutic manipulations (extrinsic).
The most frequent intrinsic conditions include prolonged hospitalizations, older age, diseases of the CNS and other chronic processes.
The most frequent extrinsic conditions include the use of artificial airways (endotracheal tubes), medication (use of sedatives, prolonged or inappropriate administration of antibiotics, prophylaxis for stress ulcer with histamine blockers and proton pump inhibitors), and the use of other tubes such as nasogastric catheters.
Preventive measures:
- 1.
Hand hygiene with washing and/or disinfection (level of evidence A-3).
- 2.
Silver-coated endotracheal tubes (B-1).
- 3.
Selective digestive decontamination (B-1).
- 4.
Oral decontamination with chlorhexidine (A-1).
- 5.
Aspiration of subglottic secretions (A-1).
- 6.
Non-invasive mechanical ventilation (NIMV) (B-1).
- 7.
Avoid changing or manipulating the respirator tubes (B-3).
- 8.
Avoid unnecessary intra-hospital transfers (B-3).
- 9.
Positional strategies (A-3).
- 10.
Other measures: daily evaluation for extubation and avoidance of reintubations (A-2); strict control of sedation (A-1); hospital education programs (B-4); avoidance of blood transfusions (B-1); rigorous disinfection of respiratory equipment (B-3); prevention of the contamination of aerosols (B-3).
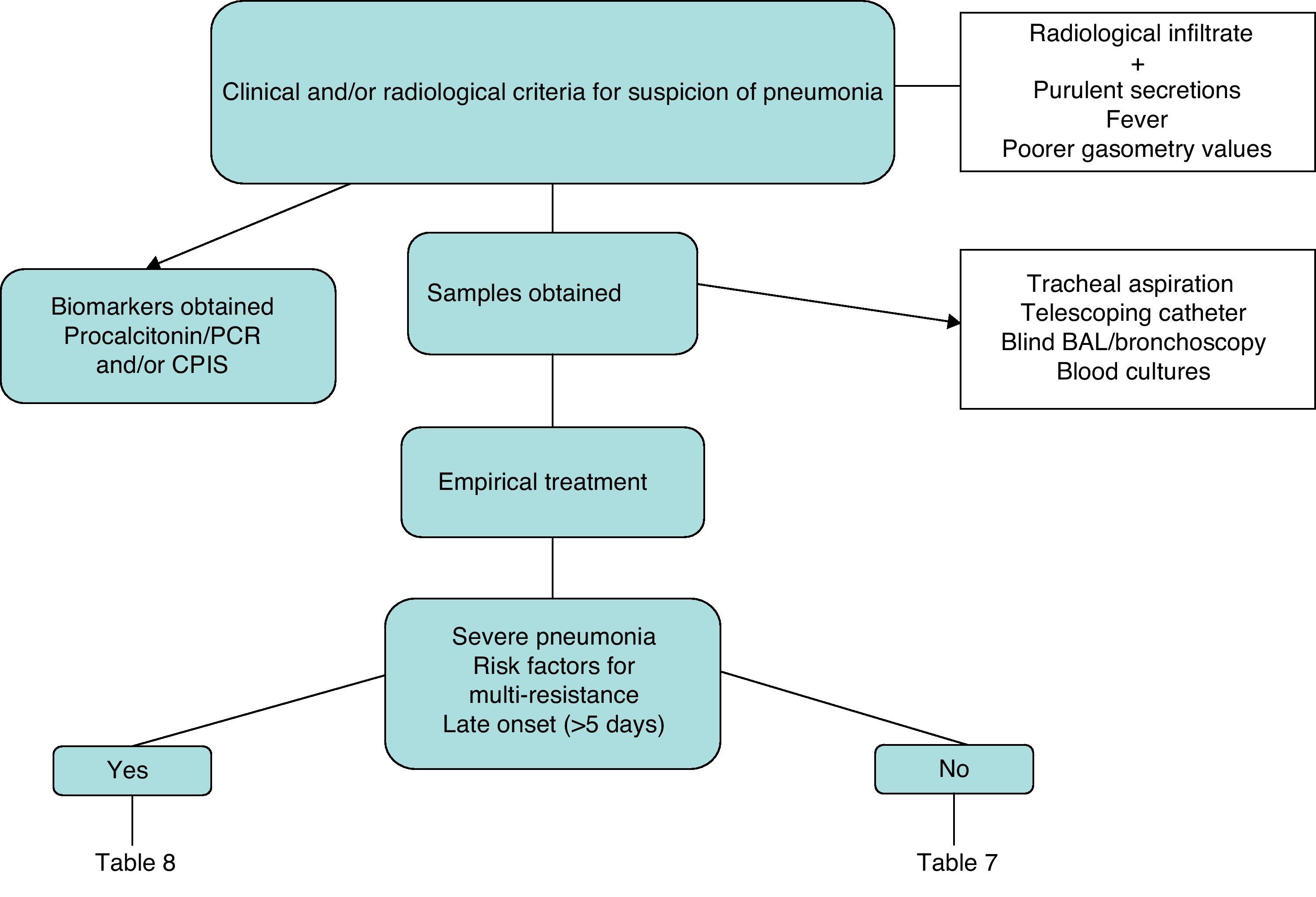
Diagnosis:
- 1.
The diagnosis of pneumonia is based on the combination of clinical and radiological data (A-1).
- 2.
It is recommended to obtain a respiratory sample, either by means of bronchoscopy or non-bronchoscopic techniques. The quantification of the number of colonies should be interpreted in the context of exposure to antibiotics, underlying pathology and period of hospitalization (A-1).
- 3.
Although the sensitivity of blood cultures in the diagnosis of nosocomial pneumonia is low, it can rule out other sources of infection; therefore, their use is recommended (A-3).
- 4.
Rapid diagnostic techniques in the respiratory sample (Gram and/or determination of intracellular microorganisms in the BAL) allow for earlier, directed treatment (A-2).
- 5.
Do not delay the start of treatment while waiting for the culture results (A-1).
Treatment:
- 1.
Obtain samples for culture before initiating the empirical treatment, if possible (A-3).
- 2.
Initiate empirical treatment as soon as possible (A-3).
- 3.
Know the microbiology and antimicrobial resistance patterns of each center (A-3).
- 4.
Stratify the patients: early-onset pneumonia with no risk factors for potentially resistant microorganisms (group 1); or, late-onset pneumonia or early-onset pneumonia with risk factors for potentially resistant microorganisms (group 2) (A-3).
- 5.
Monotherapy in group 1 pneumonia (see Table 7) (A-3).
- 6.
Combined therapies in group 2 pneumonia (see Table 8) using 2 drugs with antipseudomonal activity (A-2).
- 7.
If there is MRSA endemia, add empirical linezolid or vancomycin (A-3).
- 8.
Antibiotic de-escalation according to the results of the culture and clinical response (A-2).
Response to empirical treatment:
- 1.
The response to treatment should be evaluated three days after it is initiated (A-3).
- 2.
There is no validated definition of “lack of response” to empirical treatment (B-2).
- 3.
The reduction of the Clinical Pulmonary Infection Score (CPIS) is a positive predictive factor for good evolution (B-3).
- 4.
The earliest and most sensitive parameter for good response is the improvement in arterial oxygenation (A-3).
- 5.
Serum procalcitonin is a sensitive and specific marker for following the evolution of the patients (A-2).
- 6.
In case of lack of response, it is recommended to carry out a complete microbiological re-evaluation and consider a change in antibiotic treatment (A-3).
- 7.
It is recommended to use a systematized classification in order to evaluate the cause for the lack of response to the empirical treatment (A-3).
Nosocomial pneumonia (NP) is an inflammatory process that is infectious in origin. It is absent at the time of hospital admittance and develops more than 48h after having been hospitalized. The term “early onset” has been used to refer to NP that begins within the first 96h of the hospitalization and “late onset” for those NP that appear afterwards. The name “ventilator-associated pneumonia” (VAP) is given to the subgroup of NP that occurs in patients with artificial airways, representing more than 80% of the pneumonias acquired in intensive care units (ICU).
NP is the second most frequent hospital-originated infection while VAP is the most frequent nosocomial infection in the ICU, with an incidence of 7.6 cases per thousand days of mechanical ventilation (MV).1 NP and VAP constitute an important health problem, both for their high morbidity and mortality (especially those caused by multi-resistant microorganisms) as well as for the overload they cause in the consumption of health-care resources, and consequently the high cost.
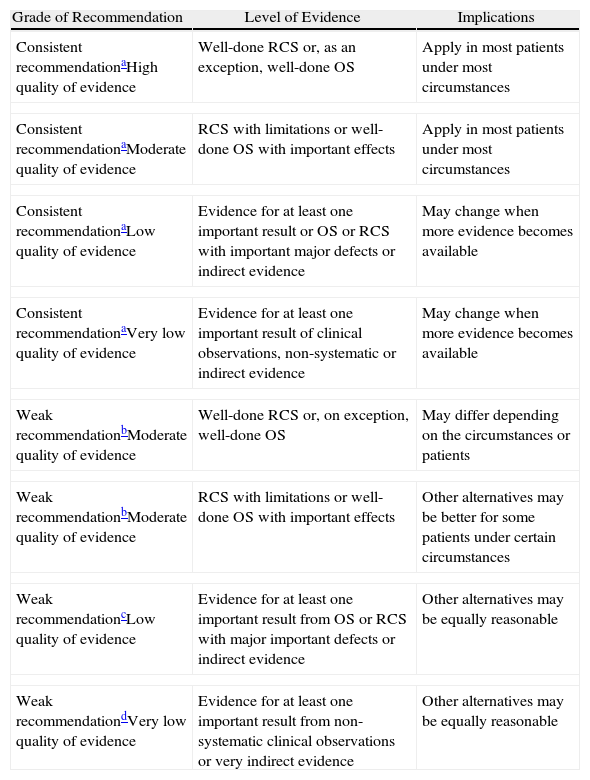
Since the publication of the latest SEPAR Guidelines for Nosocomial Pneumonia in 1997, several innovations have come about in the prevention of NP and VAP, accompanied by diagnostic and therapeutic developments. This situation makes it necessary to update the guidelines to include these recent contributions. To do so, the drafting team of these SEPAR Guidelines were divided into three subgroups in order to review the literature of the cited topics in the articles that had either been published or were in press before April 2010. Later, subsequent drafts were prepared for: (a) Pathogenesis, risk factors and prevention of NP and VAP; (b) Diagnosis of NP and VAP; and (c) Treatment and follow-up of the patients with NP and VAP. These drafts were later analyzed and approved by all the members of the writing team. The methodology used for evaluating the different levels of evidence of the recommendations has been previously described by the American Thoracic Society, based on the strength and the quality of the evidence, following the GRADE system (Grade of Recommendation, Assessment, Development and Evaluation) according to the strength of the recommendation (A: consistent recommendation, and B: weak recommendation), and levels of evidence (1: high; 2: moderate; 3: low; 4: very low) (Table 1).2
Classification of the Recommendations and Quality of Evidence According to the Grade System 2.
| Grade of Recommendation | Level of Evidence | Implications |
| Consistent recommendationaHigh quality of evidence | Well-done RCS or, as an exception, well-done OS | Apply in most patients under most circumstances |
| Consistent recommendationaModerate quality of evidence | RCS with limitations or well-done OS with important effects | Apply in most patients under most circumstances |
| Consistent recommendationaLow quality of evidence | Evidence for at least one important result or OS or RCS with important major defects or indirect evidence | May change when more evidence becomes available |
| Consistent recommendationaVery low quality of evidence | Evidence for at least one important result of clinical observations, non-systematic or indirect evidence | May change when more evidence becomes available |
| Weak recommendationbModerate quality of evidence | Well-done RCS or, on exception, well-done OS | May differ depending on the circumstances or patients |
| Weak recommendationbModerate quality of evidence | RCS with limitations or well-done OS with important effects | Other alternatives may be better for some patients under certain circumstances |
| Weak recommendationcLow quality of evidence | Evidence for at least one important result from OS or RCS with major important defects or indirect evidence | Other alternatives may be equally reasonable |
| Weak recommendationdVery low quality of evidence | Evidence for at least one important result from non-systematic clinical observations or very indirect evidence | Other alternatives may be equally reasonable |
OS: observational studies; RCS: randomized control studies.
The pathogenesis of NP is multifactorial,3 although the most frequent mechanism consists of the aspiration of microorganisms that colonize the oropharynx or the upper gastrointestinal tract.4,5 This aspiration occurs in up to 45% of healthy individuals during sleep, where there are no consequences because their oropharyngeal microbiota contains guest microorganisms. In contrast, in hospitalized individuals, the combination of a depressed immune function, the suppression of the swallowing and cough reflexes, together with the weakened clearing of the mucociliary system of the respiratory tract and the presence of comorbidities, malnutrition and pathogenic microorganisms, make aspiration a significant contributing factor to NP.3
The origin of the causal agents of the colonization and infection can be exogenous, meaning that they come from the environment (inhalation of infected aerosols, contaminated nebulizers, ventilator tubes, anesthesia equipment, bronchoscopes, hands or attire of the health-care personnel),4,6 or endogenous, meaning that they come from the usual bacterial microbiota of the patient (primary) or substituted by hospital organisms (secondary: paranasal sinuses, gastrointestinal tract, hematogenous dissemination).4,6–9 One relevant pathogenic mechanism in patients with endotracheal tubes (ET) is the formation of the bacterial biofilm,10 which is made up of bacterial aggregates. It appears within the ET and protects the organisms from the action of the antibiotics and the defenses of the patient; The microorganisms easily come loose from the biofilm with the use of suction catheters, favoring tracheal colonization and distal inoculation.6 VAP coincides in its pathogenesis with several elements: endotracheal tubes, high probability for aspiration, comorbidities and lowered defenses.8,11
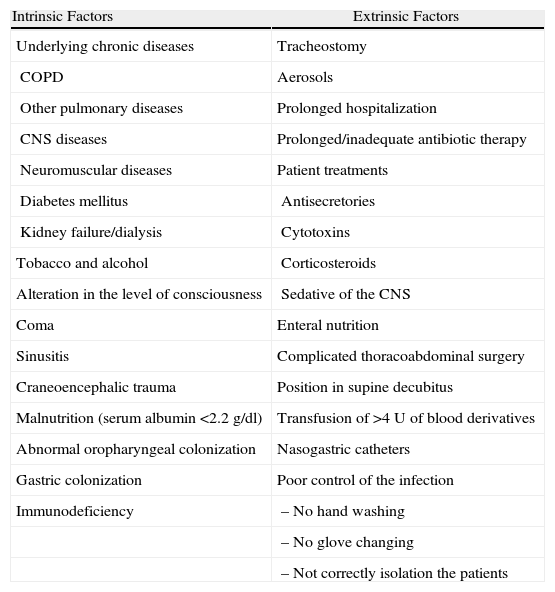
Risk FactorsClinical situations have been reported to facilitate the silent aspiration of secretions, augment the quantity and pathogenicity of the inoculated microorganisms and reduce local defenses of the respiratory tract and even systemic immunity, which are usually linked to alterations in the defense mechanisms of the host (intrinsic) and/or diagnostic–therapeutic manipulations (extrinsic)11 (Table 2). Included among these are prolonged hospitalizations, central nervous system diseases or the use of sedatives (that diminish the level of consciousness and the protective reflexes of the upper airways, or affect correct swallowing). Others include old age, uremia, the prolonged or inappropriate use of antibiotics, toxic habits (alcoholism, smoking) or rather the presence of chronic diseases (COPD, diabetes mellitus), hematologic neoplasms or treatment with chemotherapy, respiratory failure, enteral nutrition, coma, major surgery, malnutrition, multi-organ failure, together with microbiological resistance pattern to antibiotics in the community or hospital setting (Table 2), family member with a multi-resistant pathogen, neutropenia, position in supine decubitus and adult respiratory distress syndrome (ARDS). Furthermore, prophylaxis for stress ulcers with histamine blockers and proton pump inhibitors is associated with increased colonization by Gram-negative microorganisms (G−) in the digestive tract. Finally, the use of ET or nasogastric catheters interrupts the natural barriers of the lower airways, with alterations in the cough and swallowing reflexes, the glottis, and the upper and lower esophageal sphincters. Several authors have demonstrated that the duration of the endotracheal intubation and the need for reintubation or tracheostomy are risk factors for NP.1–4,9,10 So are the ET when the oropharyngeal secretions accumulate in the subglottic region, above the endotracheal tube cuff, as they have a high bacterial load that includes hospital pathogenic flora, which is a powerful inoculum during microaspiration. In the same manner, the manipulation of the respirator tubes is also a risk for NP.12
Risk Factors for Nosocomial Pneumonia.
| Intrinsic Factors | Extrinsic Factors |
| Underlying chronic diseases | Tracheostomy |
| COPD | Aerosols |
| Other pulmonary diseases | Prolonged hospitalization |
| CNS diseases | Prolonged/inadequate antibiotic therapy |
| Neuromuscular diseases | Patient treatments |
| Diabetes mellitus | Antisecretories |
| Kidney failure/dialysis | Cytotoxins |
| Tobacco and alcohol | Corticosteroids |
| Alteration in the level of consciousness | Sedative of the CNS |
| Coma | Enteral nutrition |
| Sinusitis | Complicated thoracoabdominal surgery |
| Craneoencephalic trauma | Position in supine decubitus |
| Malnutrition (serum albumin <2.2g/dl) | Transfusion of >4U of blood derivatives |
| Abnormal oropharyngeal colonization | Nasogastric catheters |
| Gastric colonization | Poor control of the infection |
| Immunodeficiency | – No hand washing |
| – No glove changing | |
| – Not correctly isolation the patients |
COPD: chronic obstructive pulmonary disease; CNS: central nervous system.
The strategies involved in the prevention of NP can be directed towards modifiable risk factors for colonization and aspiration,5,7,13–15 in addition to including interventions associated with reducing MV times.8,16 These require the need for implementing multiple strategies for reducing the risk for NP (Table 3):
- 1.
Hand hygiene with washing and/or disinfection by the health-care staff, both before and after contact with the patients. This simple maneuver, with proven effectiveness can prevent the transfer of pathogens from one patient to another and maintain the hands of the health-care personnel free from potentially pathogenic bacteria. This helps prevent cross infections and the colonization of the patients, especially if it is accompanied by the isolation of patients colonized by multi-resistant pathogens.7,17 The use of alcohol solutions has increased the compliance with hand washing (from 48% to 66%) and has reduced the rate of nosocomial infections (from 17% to 9.9%)18,19 (A-3).
- 2.
Silver-coated endotracheal tubes – In November 2007, the FDA authorized the sale of silver-coated ET, which prevent the formation of biofilm, have bactericide activity, reduce the bacterial load and reduce inflammation.8 A recent prospective, randomized, double-blind clinical assay15 with more than 2000 patients intubated for more than 24h observed that the users of these tubes had a statistically significant reduction in the incidence of VAP that was verified microbiologically (greatest impact during the first 7–10 days) and a delay in the appearance of VAP when compared with normal ET. No differences were found in the evolution of the patients either for mortality, ICU stay or duration of MV. These silver-coated ET should be considered in patients at high risk for developing VAP given their low cost and limited potential for being harmful8,20 (B-1).
- 3.
Selective digestive decontamination – This consists of the prevention of bacterial colonization, both Gram-negative as well as positive and yeast, with the topical use of non-absorbable local antibiotics in the oropharyngeal and gastrointestinal tract, either with or without parenteral antibiotics.21 Although its benefit in selected patient subgroups (surgical and trauma) has been demonstrated, its habitual use has not been recommended due to the risk for increasing antibiotic resistances, its high cost and the absence of a clear effect on mortality8,11,22 (B-1).
- 4.
Oral decontamination with chlorhexidine. This is an alternative in settings with high levels of resistances,21 and according to a recent meta-analysis,23 it is the preventive strategy with greater clinical evidence, although there are authors who doubt whether the said evidence is definitive24 (A-1).
- 5.
Aspiration of subglottic secretions – There are ET with an additional dorsal channel for the continuous or intermittent aspiration of secretions accumulated in the subglottic region.8,18,25 A meta-analysis25 demonstrated a decrease in the incidence of VAP, but not mortality, ICU stay or MV time. In the largest randomized assay done about this preventive measure,16 it was observed that in patients intubated for more than 48h, the aspiration of subglottic secretions reached significantly lower percentages of VAP, while shortening the duration of MV and ICU stay. This intervention can be used in patients at high risk in whom the duration of the MV is expected to be longer than 48h8 (A-1).
- 6.
Non-invasive mechanical ventilation (NIMV) – In selected patients, NIMV with positive pressure reduces the risk and the mortality of NP. A systematic review by Cochrane26 observed that in COPD patients NIMV reduced the risk for NP, and therefore it is recommended as an alternative for patients with acute COPD exacerbations, hypoxemic respiratory failure and those who are immunosuppressed with respiratory failure, pulmonary infiltrates and fever.5,17 NIMV is contraindicated in patients with the following characteristics: refractory hypoxemia that requires high concentrations of oxygen, hemodynamic instability or alterations in the level of consciousness. In addition, its application is difficult post-surgery (B-1).
- 7.
Avoid changes or manipulation of the respirator tubes – Prevent the unnoticed passage of condensation inside the lower airways or the nebulizers when the patient turns or when the bed is raised.27 Frequent changes in the tubes should reduce the risk for initial bacterial colonization, but well-designed studies28–30 showed that there are no advantages to changing the circuits more than once a week; in fact, more frequent change results in higher cost.24,29,31 Therefore, they should not be changed more than once a week unless they are visually contaminated with purulent secretions, vomit or blood5,25,31 (B-3).
- 8.
Avoid unnecessary intra-hospital transfers – When necessary, suspend enteral nutrition 4h before the transfer and try to place the patient semi-sitting for the transfer17,18 (B-3).
- 9.
Positional strategies – The 45° semi-sitting position reduces reflux and aspiration. It is effective, especially in patients with enteral nutrition; although probably a 30° inclination is equally effective11,13,18,32 (A-3).
- 10.
Other measures – Daily evaluation of extubation and avoid re-intubations (A-2), daily interruption of sedation (A-1), hospital education programs (B-4), avoid blood transfusions (B-1), rigorous disinfection of respiratory equipment (B-3) and prevention of contamination of aerosols (B-3).7,8,13,17,32
Recommended Measures for the Prevention of NP.
| (1) General measures that are commonly recommended |
| – Disinfection of hands with alcohol solutions |
| – Monitoring and early elimination of invasive devises: |
| - Early extubation |
| - Preference of non-invasive mechanical ventilation |
| - Avoid endotracheal intubation/re-intubation |
| – Aspiration of subglottic secretions |
| – Position of the patient inclined at 30° |
| – Avoid changes or manipulation in the respirator circuits |
| (2) Additional measures that could be useful in different settings and populations |
| - Endotracheal tubes coated with silver or with subglottic aspiration |
| - Oral decontamination with chlorhexidine |
| - Selective decontamination of the digestive tract |
| - Avoid unnecessary intrahospital transfers |
| Modified from several authors5,7,8,11,13,15,17–19,28,33 |
| Interventions in the ventilator bundle |
| 1. Pharmacological prophylaxis for stress ulcer |
| 2. Prophylaxis for deep vein thrombosis |
| 3. Elevation of the head of the bed |
| 4. Strict control of sedation |
| 5. Oral hygiene with chlorhexidine |
| 6. Daily evaluation for extubation |
| Modified from Díaz et al.18 |
The data found in the literature about the etiology of NP are numerous, although they are mostly based on observational studies, mainly in VAP. There is a wide range of microorganisms involved, where the most frequently isolated responsible agents are Pseudomonas aeruginosa and Staphylococcus aureus.33
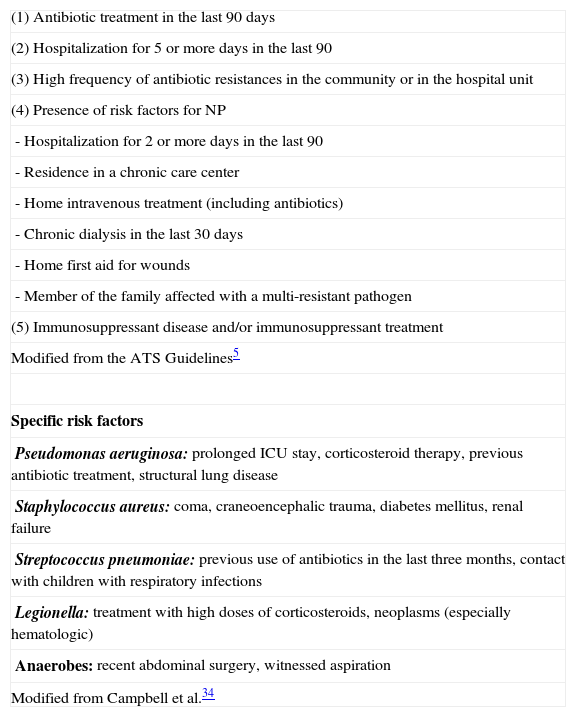
In general, it is important to identify the factors that are associated with the possibility of contracting VAP by opportunistic and multi-resistant microorganisms, as this is implicated in the treatment and prognosis. Thus, the ATS/IDSA guidelines5 differentiate between early (<5 days) and late (>5 days) pneumonia, with the objective of adjusting the treatment to the most probable etiology. In the first group, the most frequent microorganisms are Streptococcus pneumoniae and Haemophilus influenzae, while in the second there is a greater incidence of Gram-negative bacilli and multi-resistant germs. In any event, there are other factors (Table 4) that can condition the appearance of multi-resistant microorganisms during the first few days. In this regard, P. aeruginosa is especially related with the presence of COPD and the use of previous antibiotics, while MRSA entails, in addition to these factors, previous corticosteroid therapy.34 It is possible that these factors should be re-defined, as is suggested by a recent paper by Ferrer et al.35
Risk Factors for Multi-resistant Pathogens.
| (1) Antibiotic treatment in the last 90 days |
| (2) Hospitalization for 5 or more days in the last 90 |
| (3) High frequency of antibiotic resistances in the community or in the hospital unit |
| (4) Presence of risk factors for NP |
| - Hospitalization for 2 or more days in the last 90 |
| - Residence in a chronic care center |
| - Home intravenous treatment (including antibiotics) |
| - Chronic dialysis in the last 30 days |
| - Home first aid for wounds |
| - Member of the family affected with a multi-resistant pathogen |
| (5) Immunosuppressant disease and/or immunosuppressant treatment |
| Modified from the ATS Guidelines5 |
| Specific risk factors |
| Pseudomonas aeruginosa: prolonged ICU stay, corticosteroid therapy, previous antibiotic treatment, structural lung disease |
| Staphylococcus aureus: coma, craneoencephalic trauma, diabetes mellitus, renal failure |
| Streptococcus pneumoniae: previous use of antibiotics in the last three months, contact with children with respiratory infections |
| Legionella: treatment with high doses of corticosteroids, neoplasms (especially hematologic) |
| Anaerobes: recent abdominal surgery, witnessed aspiration |
| Modified from Campbell et al.34 |
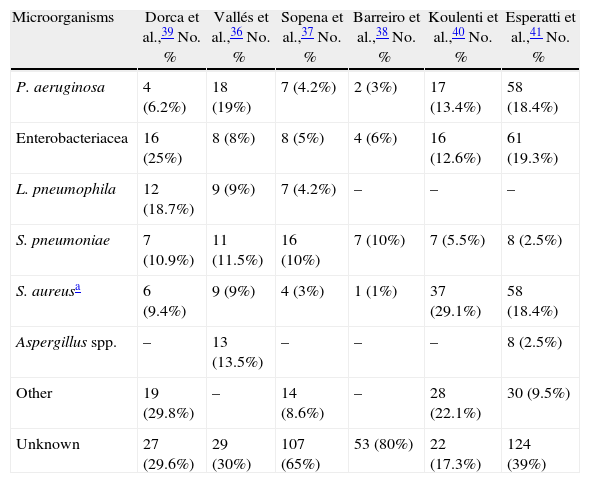
In general, and although this approximation is valid, the distribution of microorganisms causing NP varies from center to center, as can be observed in Table 5,36–41 and even it is different between units in the same hospital, therefore treatment protocols must be adapted to local circumstances.
Etiology of VAP and NAH in Several Spanish Series.
| Microorganisms | Dorca et al.,39 No. % | Vallés et al.,36 No. % | Sopena et al.,37 No. % | Barreiro et al.,38 No. % | Koulenti et al.,40 No. % | Esperatti et al.,41 No. % |
| P. aeruginosa | 4 (6.2%) | 18 (19%) | 7 (4.2%) | 2 (3%) | 17 (13.4%) | 58 (18.4%) |
| Enterobacteriacea | 16 (25%) | 8 (8%) | 8 (5%) | 4 (6%) | 16 (12.6%) | 61 (19.3%) |
| L. pneumophila | 12 (18.7%) | 9 (9%) | 7 (4.2%) | – | – | – |
| S. pneumoniae | 7 (10.9%) | 11 (11.5%) | 16 (10%) | 7 (10%) | 7 (5.5%) | 8 (2.5%) |
| S. aureusa | 6 (9.4%) | 9 (9%) | 4 (3%) | 1 (1%) | 37 (29.1%) | 58 (18.4%) |
| Aspergillus spp. | – | 13 (13.5%) | – | – | – | 8 (2.5%) |
| Other | 19 (29.8%) | – | 14 (8.6%) | – | 28 (22.1%) | 30 (9.5%) |
| Unknown | 27 (29.6%) | 29 (30%) | 107 (65%) | 53 (80%) | 22 (17.3%) | 124 (39%) |
36–39: patients hospitalized in a conventional ward. 40: VAP (data in Spain in a multicenter European study).
41: ventilated and non-ventilated patients in ICU.
The clinical diagnosis is based on the combination of a newly appearing radiological infiltrate together with purulent secretions (except in neutropenia), and one or more of the following criteria: fever, hypoxemia or leukocytosis. Despite this, the clinical symptoms are unspecific in mechanically ventilated patients and can be confused with other entities, such as atelectasis, pulmonary thromboembolism and sepsis of other origins.5 In a recent review42 evaluating clinical criteria (including only studies that use the histologic findings as a reference), it was concluded that the presence of two clinical criteria (fever, leukocytosis or purulent secretions) together with one radiological criterion (newly appearing opacity) increases the probability of having pneumonia by 2.8 (95% CI, 0.97–7.9), while the absence of radiological infiltrate reduces the probability to 0.35 (95% CI, 0.14–0.87).
There are episodes compatible with lung infection and isolation of microorganisms in significant concentrations in ventilated patients that do not present visible pulmonary infiltrate, known as respirator-associated tracheobronchitis. Although it has not often been analyzed, according to the most recent studies this entity seems to increase the days of MV and even leads to mortality if it is not treated with antibiotics.43 Still it has yet to be defined whether it represents a true VAP (lack of radiological resolution) or simply a precursor to VAP.
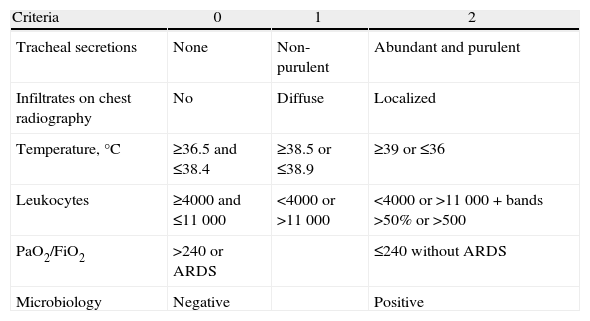
With the aim to improve the sensitivity and the specificity of the diagnosis of pneumonia, Pugin et al.44 developed a predictor scale, the Clinical Pulmonary Infection Score (CPIS) (Table 6), which evaluates a series of parameters (temperature, leukocyte count, appearance of respiratory secretions, oxygenation, chest radiography, Gram stain and tracheal aspiration culture). Scores higher than 6 were associated with the diagnosis of pneumonia in the original series, where the sensitivity and specificity were 93% and 100%, respectively. However, using the histopathological findings as a reference, Fàbregas et al.45 observed a sensitivity of 77%, but a specificity of only 42%.
Clinical Pulmonary Infection Score, Made up of 6 Items With a Score Ranging From 0 to 12.
| Criteria | 0 | 1 | 2 |
| Tracheal secretions | None | Non-purulent | Abundant and purulent |
| Infiltrates on chest radiography | No | Diffuse | Localized |
| Temperature,°C | ≥36.5 and ≤38.4 | ≥38.5 or ≤38.9 | ≥39 or ≤36 |
| Leukocytes | ≥4000 and ≤11000 | <4000 or >11000 | <4000 or >11000+bands >50% or >500 |
| PaO2/FiO2 | >240 or ARDS | ≤240 without ARDS | |
| Microbiology | Negative | Positive |
Microbiological testing in NP includes the qualitative and quantitative analysis of the respiratory secretions obtained using bronchoscopic (directed) or non-bronchoscopic (blind) techniques, or also by taking tracheal aspiration samples. The first two can be done by bronchoalveolar lavage (BAL) and protected telescoping catheter (PTC), while the latter consists of taking secretions directly through the ET.
Obtaining respiratory samples is a common practice in the diagnostic process of VAP, but the validity of said diagnosis is questioned if it is only based on the microbiological data. In this sense, the quantification of the bacterial load does not have a documented scientific basis46; thus, in BAL and tracheal aspiration, the diluted volume is not standardized. Moreover, the techniques used in BAL are diverse, with disparate results when the test is repeated.46 Thus, the cut-points below those recommended do not exclude the existence of pneumonia, as said points only represent the probability of pneumonia.47
Both in the qualitative as well as in the quantitative analysis, it is important to evaluate the quality of the respiratory samples. The finding of more than 1% squamous cells in BAL or PTC represents a clear oropharyngeal contamination; on the other hand, the existence of less than 10% of neutrophils makes the diagnosis of pneumonia very unlikely. In bronchial aspiration samples, a quality sample should have at least 10% squamous cells.
Blood CulturesIn general, these are not very sensitive (less than 20%, and in ventilated patients, around 8%). However, and although a positive isolation does not confirm the pulmonary origin, blood cultures are indicated in patients with suspicion for VAP as it has prognostic implications and positive blood cultures are more frequently associated with methicillin-resistant S. aureus (MRSA).48,49
Bronchoscopic vs Non-bronchoscopicThe need to avail/employ an experienced bronchoscopist, the costs and the minimization of risks for the patient have caused a dawning of non-bronchoscopic techniques, such as a blind telescoping catheter or BAL catheters that obtain samples easily and reproducibly. Despite the intense debate about the superiority of one technique over the others, it seems that there are no significant differences in the sensitivity and specificity between the two types of techniques. A detailed review on this topic concluded that the sensitivity of blind techniques is from 30% to 70%, and the specificity from 90% to 100%.50 Likewise, in the case of BAL, there is the possibility of using volumes lower than recommended (100–150ml saline solution), without altering the diagnostic precision.
Tracheal AspirationThis is the most simplified way to obtain samples. In recent years, five randomized trials have been done comparing bronchoscopic techniques with qualitative tracheal aspiration. In a recent review analyzing these studies, it was concluded that there are no significant differences regarding mortality or the use of antibiotics.51
In clinical practice, tracheal aspiration is more frequently used. In a multicenter study carried out in 9 European countries, bronchoscopic techniques were used in less than 20% of patients, while quantitative tracheal aspiration represented 70% of the cases.40
Direct Microbiological Testing of Respiratory SamplesThe quantification of intracellular microorganisms is a technique that enables early diagnosis and the possibility to initiate directed treatment. Along these lines, the determination in BAL of more than 2% of intracellular microorganisms has a positive predictive value of close to 100%.52
As for the Gram determination in samples from aspirations and PTC, there are fewer published experiences, with diverging results. In the study with a greater number of patients, the kappa agreement rate between the Gram reading and the final result of the culture was only 0.36.53
BiomarkersIn recent years, several biomarkers have been used with the intention of improving the sensitivity and the specificity in the diagnosis of VAP; among the most widely studied are procalcitonin (PCT), C-reactive protein (CRP) and sTREM-1. Although the initial publications54 with sTREM-1 were promising, with sensitivities and specificities close to 100%, these were later unable to be confirmed. Thus, in a recent study, choosing a sensitivity of 95%, the positive predictive value (PPV) was 41% and the negative predictive value (NPV) was 62% in the population studied. With a specificity of 95%, the PPV was 67% and the NPV 62%.55
Regarding CRP and PCT, their diagnostic usefulness has only been determined in a study done with 44 patients. The determination of procalcitonin showed a sensitivity of 78% and a specificity of 97%, while the CRP values were 56% and 91%, respectively.56
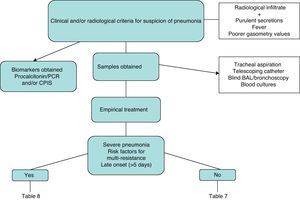
Fig. 1 is a diagnostic algorithm for NP that includes clinical–radiological, biological and microbiological criteria.
TreatmentGeneral RecommendationsIn patients with suspicion for NP and VAP, the samples for microbiological studies should be collected as soon as possible. Meanwhile, however, the start of empirical treatment should not be delayed due to the need for performing special procedures. A fundamental aspect at this moment is to ensure that said initial treatment is appropriate and adequate. An “appropriate” empirical treatment refers to the use of an antibiotic to which the possible etiological microorganism(s) are sensitive. “Adequate” treatment refers to the use of an appropriate antibiotic at the correct dosage, with good penetration at the site of the infection and, when indicated, in a combination. Several studies have demonstrated the importance of initiating appropriate empirical antibiotic treatment from the start.57 The correction of an inappropriate initial treatment according to the results of the respiratory secretion cultures does not reduce the accompanying mortality; therefore, all efforts should be aimed at ensuring that the initial treatment is appropriate and adequate.58 With the objective of implementing an appropriate empirical treatment, it is of vital importance to know the microbiology of the hospital itself and for each hospitalization unit59 and follow the recommendations of the treatment guidelines developed by the scientific societies in this field.
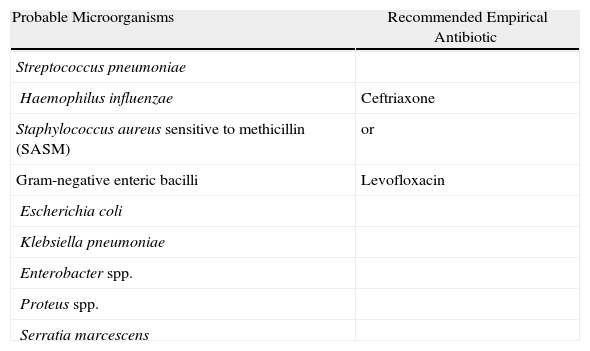
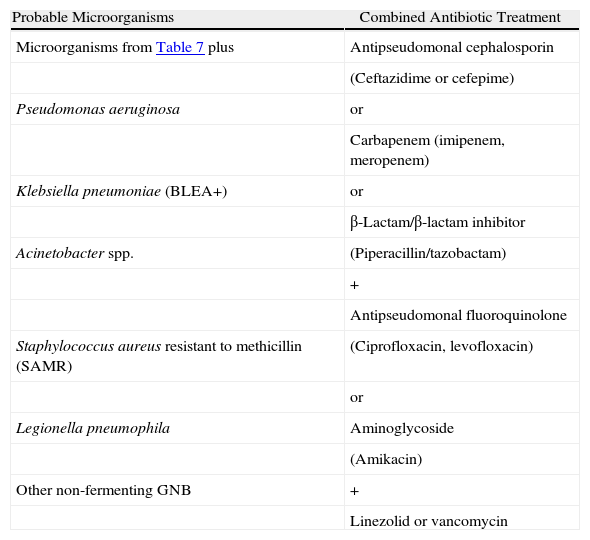
Stratification of the Patients and Recommendations for the Empirical TreatmentThe American Thoracic Society5 published some guidelines for the diagnosis and treatment of adult patients with NP in which it is considered that the two main factors that determine the type of antibiotics to be administered are the time that the patient has been hospitalized, classifying the pneumonia as early (<5 days) or late (≥5 days), and the presence of risk factors for infection by potentially multi-resistant microorganisms (MRMO) (Table 4). In patients with early-onset NP and without risk factors for MRMO, the treatment should cover pathogens that are generally found in the community and with low probability of multi-resistances (Table 7). On the contrary, the patients with late-onset NP or with presence of risk factors for MRMO should receive a wide-spectrum initial empirical treatment, administered in combination in order to guarantee the coverage of the majority of causal microorganisms in this group of patients (Table 8). The objective of the use of combined treatment is to find the synergy between different antibiotic groups, widening the spectrum to ensure an appropriate treatment against Gram-negative microorganisms, and avoid the development of resistances. Both the antibiotic dose and the recommended intervals are shown in Table 9.
Early-onset NP and VAP, Without Risk Factors for Infection by Multi-resistant Pathogens and Any Stage of Severity.
| Probable Microorganisms | Recommended Empirical Antibiotic |
| Streptococcus pneumoniae | |
| Haemophilus influenzae | Ceftriaxone |
| Staphylococcus aureus sensitive to methicillin (SASM) | or |
| Gram-negative enteric bacilli | Levofloxacin |
| Escherichia coli | |
| Klebsiella pneumoniae | |
| Enterobacter spp. | |
| Proteus spp. | |
| Serratia marcescens |
Late-onset NP and VAP, With Risk Factors for Multi-resistant Germs of Any Degree of Severity.
| Probable Microorganisms | Combined Antibiotic Treatment |
| Microorganisms from Table 7 plus | Antipseudomonal cephalosporin |
| (Ceftazidime or cefepime) | |
| Pseudomonas aeruginosa | or |
| Carbapenem (imipenem, meropenem) | |
| Klebsiella pneumoniae (BLEA+) | or |
| β-Lactam/β-lactam inhibitor | |
| Acinetobacter spp. | (Piperacillin/tazobactam) |
| + | |
| Antipseudomonal fluoroquinolone | |
| Staphylococcus aureus resistant to methicillin (SAMR) | (Ciprofloxacin, levofloxacin) |
| or | |
| Legionella pneumophila | Aminoglycoside |
| (Amikacin) | |
| Other non-fermenting GNB | + |
| Linezolid or vancomycin |
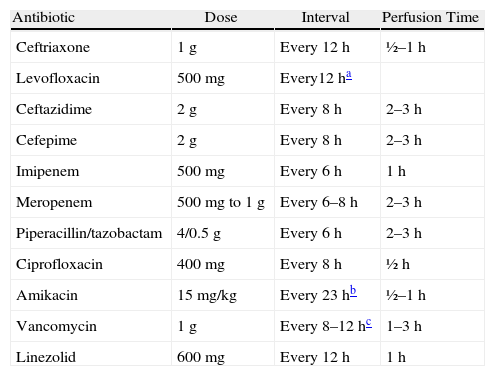
Recommended Doses and Intervals for the Main Antibiotics Recommended in the Treatment for Nosocomial Pneumonia and Associated With Mechanical Ventilation.
| Antibiotic | Dose | Interval | Perfusion Time |
| Ceftriaxone | 1g | Every 12h | ½–1h |
| Levofloxacin | 500mg | Every12ha | |
| Ceftazidime | 2g | Every 8h | 2–3h |
| Cefepime | 2g | Every 8h | 2–3h |
| Imipenem | 500mg | Every 6h | 1h |
| Meropenem | 500mg to 1g | Every 6–8h | 2–3h |
| Piperacillin/tazobactam | 4/0.5g | Every 6h | 2–3h |
| Ciprofloxacin | 400mg | Every 8h | ½h |
| Amikacin | 15mg/kg | Every 23hb | ½–1h |
| Vancomycin | 1g | Every 8–12hc | 1–3h |
| Linezolid | 600mg | Every 12h | 1h |
A meta-analysis about the use of beta-lactams, alone or in combination with aminoglycosides for the treatment of sepsis in immunocompetent patients,60 was not able to demonstrate a beneficial effect in terms of mortality, clinical or microbiological failure in the complete group of patients, nor for mortality in the subgroup of patients infected by P. aeruginosa. Nevertheless, the study found a greater nephrotoxicity of the therapy combined with aminoglycosides. However, in another meta-analysis that evaluated the role of combined treatment in patients with bacteriemia due to Gram-negative bacilli, the authors found a beneficial effect of the cited treatment only in the subgroup of patients infected by P. aeruginosa.61 The combined treatment is administered using an antipseudomonal beta-lactam (third or fourth generation cephalosporin, penicillin associated with an inhibitor of beta-lactamase or carbapenems) combined with an aminoglycoside or a quinolone active against Pseudomonas. In cases with satisfactory evolution, the aminoglycoside or the quinolone can be suspended after 5 days of combined treatment.
When there are multi-resistances and few possibilities for antibiotic combinations, as can be the case of pneumonias due to P. aeruginosa and Acinetobacter baumanii, the effectiveness of the administration of nebulizer antibiotic therapy has been confirmed when added to the intravenous antibiotic treatment.62 The antibiotics that have been used are aminoglycosides and colistin. More experience is required regarding its dose, pulmonary penetration and the secondary effects of the use of antibiotics administered in this manner.
As for the treatment of tracheobronchitis associated with the respirator, a randomized study with few patients shows a lower mortality in the patients who received antibiotic treatment.43 While waiting for later studies to confirm this, the recommendation would be to treat these patients with the same therapeutic treatment used for NP.
Duration of the TreatmentTraditionally, the duration of antibiotic treatment was between 7 and 10 days for early nosocomial pneumonia, which are caused by microorganisms that are generally sensitive and found in the community. For late pneumonias, the recommendations considered treatment times of up to 21 days in patients infected with multi-resistant bacteria like P. aeruginosa and A. baumanii. However, in current clinical practice the duration of the treatment is being shortened, based on clinical studies. The most important study, published by Chastre et al.,63 evaluates prospectively, randomly and double-blind two different therapies in patients with VAP, carried out in various ICUs in France. The main objective was to compare an 8-day cycle of antibiotic treatment with another 15-day cycle. They randomized 401 patients (197 to the 8-day treatment group and 204 the 15-day group); after 28 days, mortality was 18.8% in the 8-day group vs 17.2% in the 15-day treatment group. There were also no differences regarding days of MV, severity, days of organ failure or presence of bacteriemia, ARDS or shock. The recurrence rate of a microbiologically documented lung infection was 28.9% in patients treated for 8 days vs 26% in those treated for 15 days. Nevertheless, when they analyzed the recurrences of the subgroup of patients with primary infections caused by non-fermenting Gram-negative bacilli, this rate was significantly higher in the patients of the 8-day group (40.6% vs 25.4%), without involving differences in mortality. Thus, in short, it seems prudent to limit the treatment to 7–10 days in early nosocomial pneumonia, and lengthening said therapy to a minimum of 14 days in cases of late pneumonia, especially those caused by multi-resistant bacteria, both Gram-negative (P. aeruginosa, A. baumanii) as well as Gram-positive (S. aureus resistant to methicillin).
De-escalationA strategy currently used in clinical practice that is also the object of several studies is the so-called de-escalation or reduction therapy.64 This consists of a reduction in the spectrum or number of antibiotics based on the results of microbiological cultures. In various studies, this strategy has achieved a decrease in the use of antibiotics, without a significant increase in the rate of recurrences or mortality.65
Response to Empirical TreatmentOnce the microbiological results are available, the empirical treatment can be modified if resistant or unexpected pathogens are isolated in a patient who does not respond to treatment. On the other hand, if no pathogens are isolated or these are sensitive to antibiotics of a smaller spectrum, these can be reduced or even withdrawn.
Normal Pattern of ResolutionClinical concepts such as improvement, resolution, differed resolution, relapse, failure and death are well-defined.66 The clinical improvement generally becomes evident in the first 48–72h of treatment; therefore, the antimicrobial treatment should not be changed during this period, unless progressive deterioration is observed or the initial cultures indicate it.66,67
Serial cultures of respiratory samples can establish the microbiological response. These can define microbial eradication, superinfection, recurrent infection and persistence.68 It is recommendable to repeat the microbiological cultures 72h after initiating treatment, as there is a good correlation between clinical failure and isolation of pathogens at significant concentrations in the follow-up.69
The radiological evolution has limited value. An initial radiological deterioration is common, especially in patients with bacteriemia or highly virulent organisms. Furthermore, the radiological improvement is usually slower than the clinical parameters.67 Nevertheless, an increase higher than 50% of the size of the infiltrate after 48h, with multi-lobar affectation, cavitation or significant pleural effusion, should be considered a sign of alarm.5
Together with microbiology, the most reliable parameters for defining the resolution of NP are leukocyte count, oxygenation and central temperature. In patients with adequate initial treatment, these parameters improve during the first week of treatment.68 There is also a good correlation between the evolution of the Clinical Pulmonary Infection Score (CPIS) (Table 6) in the first 3 days of treatment with regards to the adequacy of the empirical treatment and survival.69
The serial determination of C-reactive protein (CRP)70–72 and procalcitonin56,62,72 can aid in the decision to interrupt or modify the antimicrobial treatment. Specifically, serial procalcitonin helps, together with clinical parameters, to detect the response to treatment in NP,62 and it could also be useful in the de-escalation or suspension of antibiotic treatment.73
It has been proposed to define the lack of response to empirical treatment according to one of the following criteria in the first 72h of treatment: (1) no improvement in oxygenation or need for tracheal intubation; (2) persistence of fever or hypothermia together with purulent secretions; (3) increase in radiological lung infiltrates ≥50%; or (4) appearance of septic shock or multi-organ dysfunction.74 This definition, however, is pending clinical validation. Likewise, the evolution of the inflammatory markers and CPIS can be of great help.
Causes of Deterioration or Lack of Response to Empirical TreatmentThere is an estimated incidence of 20%–60% of lack of response to initial treatment, depending on the severity and the comorbidities, which is associated with a poor prognosis.57,74–76 Age, the previous duration of MV, neurological disease and lack of improvement in oxygenation by the third day of treatment are risk factors associated with clinical failure in VAP.77
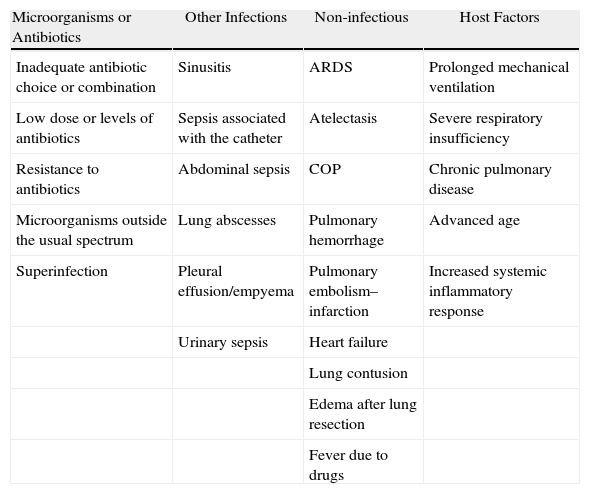
There are many possible causes of a rapid deterioration or absence of improvement in patients with clinical suspicion for NP (Table 10).
Possible Causes for the Lack of Clinical Response to Initial Antibiotic Treatment.
| Microorganisms or Antibiotics | Other Infections | Non-infectious | Host Factors |
| Inadequate antibiotic choice or combination | Sinusitis | ARDS | Prolonged mechanical ventilation |
| Low dose or levels of antibiotics | Sepsis associated with the catheter | Atelectasis | Severe respiratory insufficiency |
| Resistance to antibiotics | Abdominal sepsis | COP | Chronic pulmonary disease |
| Microorganisms outside the usual spectrum | Lung abscesses | Pulmonary hemorrhage | Advanced age |
| Superinfection | Pleural effusion/empyema | Pulmonary embolism–infarction | Increased systemic inflammatory response |
| Urinary sepsis | Heart failure | ||
| Lung contusion | |||
| Edema after lung resection | |||
| Fever due to drugs |
ARDS: acute respiratory distress syndrome; COP: cryptogenic organizing pneumonia.
The causal pathogen can be resistant to the chosen antibiotic, acquire resistance during treatment or be inherently difficult to eradicate. The pathogens most frequently associated with the lack of response and poor prognosis are Gram-negative bacilli (P. aeruginosa, Acinetobacter sp., Enterobacter sp. and Klebsiella pneumoniae, producer of wide-spectrum beta-lactamase) and S. aureus resistant to methicillin. There are also uncommon pathogens that are outside the spectrum of empirical treatment (Mycobacterium tuberculosis, fungi or respiratory viruses). Some patients can have unknown immunodeficiency (acquired immunodeficiency syndrome) and pathogens like Pneumocystis jirovecii.
Other InfectionsOther simultaneous causes of non-pneumonic fever include sinusitis, infection of the vascular catheter, pseudomembranous colitis, abdominal sepsis or urinary infections.78,79 Some complications of pneumonia can contribute to therapeutic failure, such as the development of a lung abscess or empyema. One must also consider fever due to medication or sepsis with multi-organ failure.
Non-infectious CausesThese are processes misdiagnosed as NP, such as atelectasis, heart failure, pulmonary embolism with infarction, lung contusion, organizing pneumonia, aspiration and acute respiratory distress (ARDS). Pulmonary hemorrhage, sometimes associated with pneumonia, is frequent in ventilated patients.
Host FactorsThese include conditions associated with mortality, like prolonged MV, respiratory failure, severe underlying disease (especially chronic respiratory), older age and bilateral radiological infiltrates at the onset of the pneumonia, as well as the increased systemic inflammatory response, detected by means of high levels of proinflammatory cytokines.62,74,76
Evaluation of Patients Who Do Not Respond to Empirical TreatmentIt is fundamental to obtain respiratory samples for culture, as long as it is possible by bronchoscopy (or tracheal aspiration in intubated patients), as well as blood cultures.74,75 In patients with rapid deterioration or those who do not respond to the initial treatment, the antimicrobial coverage may be extended until the results of the cultures or other studies are known. If there are resistant or uncommon pathogens, the treatment should be modified. If said pathogens are not isolated, another previously described process should be considered.
Chest radiography in lateral decubitus, ultrasound or computed tomography (CT) can detect or rule out pleural effusion (in such cases, empyema must be excluded), parenchymatous abscesses or lymphadenopathies, and can also identify other foci of infection. In intubated patients with nasogastric catheters, CT can show evidence of a sinus infection that coexists with the NP.23
If the microbiological and radiological studies are negative, different approaches may be decided: (1) observation, maintaining or changing the antibiotic treatment; (2) continue with the diagnostic study in order to identify extrapulmonary infectious foci; (3) carry out bronchoalveolar lavage in order to look for less common or opportunistic organisms. If these explorations are negative, surgical lung biopsy may be considered to identify non-infectious processes that could mimic pneumonia. The value of the pulmonary biopsy is debated due to lack of evidence that suggests a clear benefit. Therefore, the decision should be individualized for each patient.
If the improvement is slow but progressive, it is better to maintain the patient under observation. If he/she remains hemodynamically stable but without clinical improvement and the radiological explorations as well as the bronchoscopy are anodyne, the antibiotic treatment may be modified or anti-inflammatory treatment with corticosteroids can be considered before performing a pulmonary biopsy.
Conflict of InterestsA. Anzueto was a researcher at BARD in the study on endotracheal tubes. J. Rello is a consultant and lecturer for Astellas, Pfizer, Jansen and Kenta.
A. Torres is a consultant for Astellas, MSD and Sanofi Pasteur, lecturer for Astellas, MSD, Pfizer, and has received research grants from Pfizer and Covidien.