Bronchial thermoplasty (BT) is a new endoscopic treatment for severe and uncontrolled asthma patients despite adequate medical treatment.1 BT applies radiofrequency (RF)-produced heat to the bronchial wall. Clinical trials have observed improvement in quality of life and fewer exacerbation rates.2 The proposed mechanism for improvement in asthma control is the reduction in the amount of airway smooth muscle (ASM) and consequent reduction in the airway hyperresponsiveness. Reduction of ASM induced by BT has been observed in animal models3 and small case series.4–6
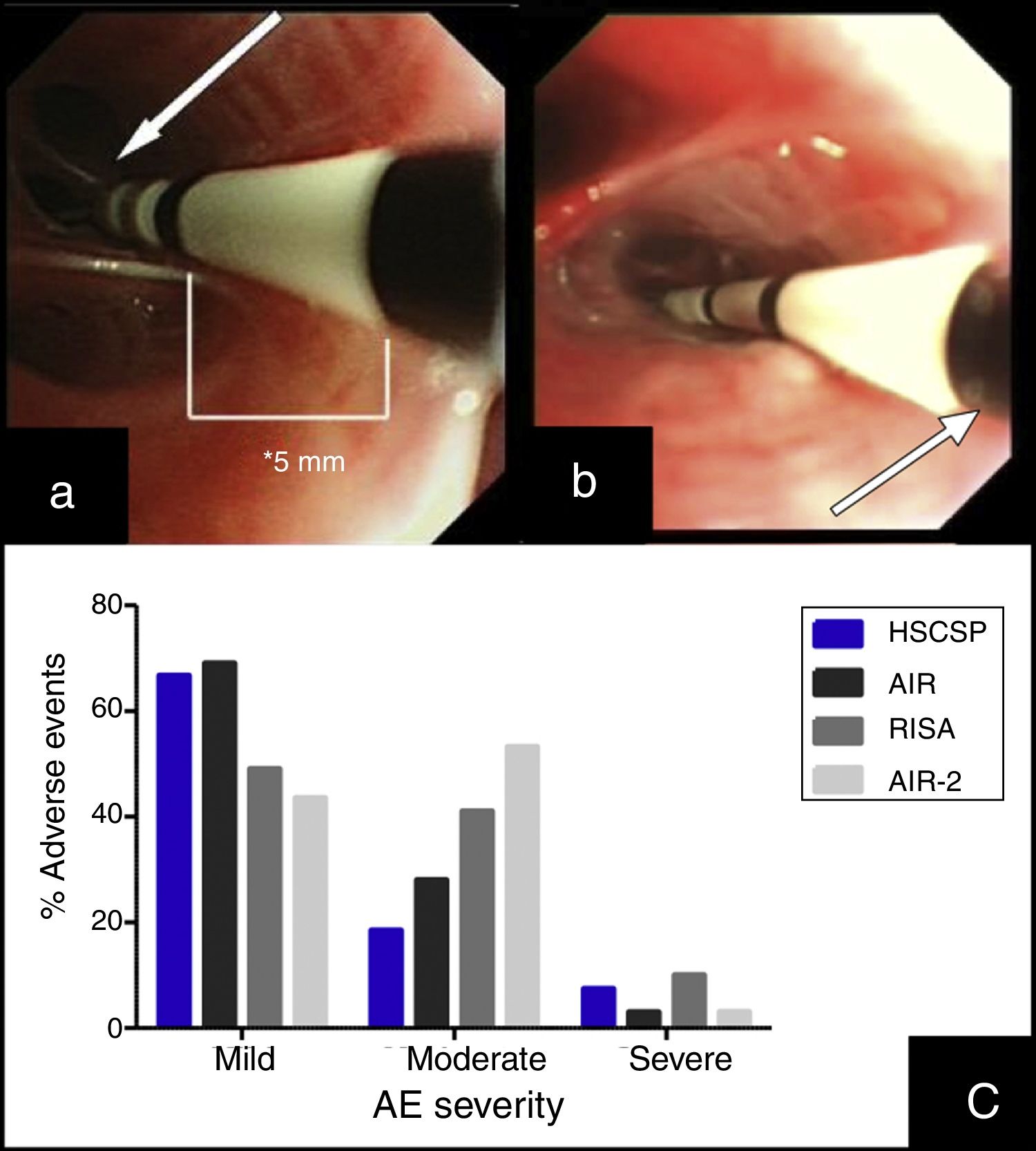
BT treatment requires three different bronchoscopy sessions (right lower lobe (RLL), left lower lobe (LLL) and upper lobes (UL), respectively) separated each other by a minimum of 3 weeks. The catheter is introduced into the working channel of the bronchoscope and placed in visible airways from distal to proximal guided by 5mm distance marks. The technique description advices not to lose visual contact with the distal extreme of the catheter, where the electrodes are placed (Fig. 1(a)).7 The amount of bronchial mucosa treated is limited to accessible airways where the tip of the catheter can be observed from bronchoscopic vision.
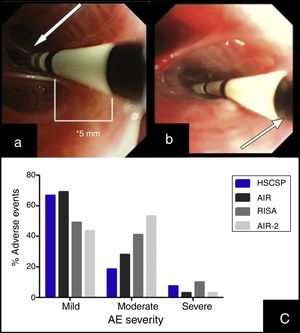
(a) Insertion of the catheter up to distal airway without losing visual contact with the electrodes (arrow) according to established protocol. (b) Introduction of the catheter with loss of visual contact with the electrodes wire, but not with the last black mark (arrow), located at 2cm from the electrodes wire. (c) Occurrence of adverse events in different series: our Hospital de la Santa Creu I Sant Pau are the sum of AE observed during the procedure and the 24h after (percentages are calculated from the 27 modified procedures); AIR: Asthma Intervention Research9; RISA; Research in Severe Asthma10; AIR-2.11
Considering BT as a locally applied treatment, there is controversy whether the number of activations and the extension of the treated area might have an influence in clinical response. Some authors suggest that the thermoplasty effect might be also related to regional changes induced by neurogenic mechanisms while others found that higher number of activations were related to better response to BT, measured by the Asthma Control Questionnaire Score (ACQ-5).8
This article describes a little modification in the published protocol, allowing further introduction of the catheter into smaller airways, losing visual contact with the distal extreme of the catheter but not with the last black mark, placed at 2cm of the electrode wire (Fig. 1(b)). We hypothesized that this modification might increase the treated bronchial area (at least 2cm each bronchus) and thus might have a potential benefit in the clinical response without increase in side effects.
Approval for this study was obtained by the Clinical Research Ethics Committee of our center (approval number: EC/12/103/1388) and patients included signed inform consent. We included all severe and uncontrolled asthma patients1 accepted for BT by an expert asthma team in our center since September 2012. Other inclusion criteria were: >18 years old, uncontrolled disease (maintained Asthma Control Test [ACT] score <19; ≥2 exacerbations in the previous year) and impaired quality of life (maintained shorter version of the Asthma Quality of Life [AQLQ] score <6.5). Subjects were excluded if: contraindication for bronchoscopy, allergy to sedative drugs, implantable devices, current smokers, past smoking habit of >15 pack-years, other respiratory diseases such as emphysema or bronchiectasis, concomitant non-respiratory diseases, such as severe cardiopathies, that could contribute to impaired control of asthma.
BT was performed with the modified procedure in three different sessions. Patients were deeply sedated with remiphentanyl and propofol, and intubated (Bronchoflex 7.5mm, Rüsch, Teleflex Medical, Durham, NC, USA) to achieve a better control of cough.
We collected the length of the procedure, number of activations, adverse events (AE) during each BT session and in the first 24h after. AE were classified as described previously10 into: mild (transient symptoms well tolerated not interfering with normal activities, and that did not required treatment except by short-acting bronchodilators; bleeding during bronchoscopy that did not required any specific measure for its resolution); moderate (symptoms that caused interference with patient's usual activities; symptomatic treatment is possible; bleeding during bronchoscopy that requires aspiration during >3s); and severe (signs or symptoms causing inability to do work or usual activities, requiring medical intervention and/or treatment; bleeding during bronchoscopy that leads to stop the procedure).
We included 9 patients (8 women, 1 male; mean age 50±17.11 years; post-bronchodilator FEV1 82±15%). All patients were in step 5 of treatment.1 In order to prevent the AE related to BT, all patients received 50mg/day prednisone (or equivalent) the 3 days before and the day after the procedure. BT treatment was completed in all patients (27 procedures).
The mean number of activations per procedure was 76.52±31.26 (71.66±19.79 in RLL, 64.55±14.99 in LLL and 93.37±47.45 in UL) with a mean length of 65.81±19.32min (64.3±18.58 in RLL, 59.2±14.14 in LLL and 73.88±23.28 in UL).
During the procedure, 7 patients suffered mild AE (6 bleeding and 1 bronchoespasm). In the 24h post-procedure we observed AE in 18 procedures. Most of them were mild (11/27) and moderate (5/27), consisting in cough and unspecific chest discomfort. Two patients had severe AE: one case of severe bronchospasm and acute respiratory insufficiency, and one case of collapse of the treated lobe with intense hypoxemia due to mucous plug. We did not find significant differences regarding adverse events when comparing our sample with those reported previously in clinical trials (Fig. 1(c)).10,11 No deaths were occurred, and all severe AE resolved.
This little change in the procedure of BT allowed us to treat an extended bronchial area, as it is shown by the mean number of applications in our patients, which is higher than the average number of applications reported previously.12 The extended treated bronchial area did not increase adverse effects. Further research is needed to know long-term safety and whether this technical modification might increase the clinical benefits.
This medical research was supported by a grant from the Sociedad Española de Neumología y Cirugía Torácica (SEPAR, 2012), a grant from Asociación Española de Endoscopia Respiratoria (AEER, 2013), and a prize from the Fundació Catalana de Pneumologia (FUCAP, 2014).