Scales for predicting venous thromboembolism (VTE) recurrence are useful for deciding the duration of the anticoagulant treatment. Although there are several scales, the most appropriate for our setting has not been identified. For this reason, we aimed to validate the DASH prediction score and the Vienna nomogram at 12 months.
MethodsThis was a retrospective study of unselected consecutive VTE patients seen between 2006 and 2014. We compared the ability of the DASH score and the Vienna nomogram to predict recurrences of VTE. The validation was performed by stratifying patients as low-risk or high-risk, according to each scale (discrimination) and comparing the observed recurrence with the expected rate (calibration).
ResultsOf 353 patients evaluated, 195 were analyzed, with an average age of 53.5±19 years. There were 21 recurrences in 1 year (10.8%, 95% CI: 6.8%–16%). According to the DASH score, 42% were classified as low risk, and the rate of VTE recurrence in this group was 4.9% (95% CI: 1.3%–12%) vs. the high-risk group that was 15% (95% CI: 9%–23%) (P<.05). According to the Vienna nomogram, 30% were classified as low risk, and the rate of VTE recurrence in the low risk group vs. the high risk group was 4.2% (95% CI: 0.5%–14%) vs. 16.2% (95% CI: 9.9%–24.4%) (P<.05).
ConclusionsOur study validates the DASH score and the Vienna nomogram in our population. The DASH prediction score may be the most advisable, both because of its simplicity and its ability to identify more low-risk patients than the Vienna nomogram (42% vs. 30%).
Las escalas predictivas de recurrencias de ETV son útiles para decidir la duración del tratamiento anticoagulante. Aunque hay varias escalas, desconocemos la aplicabilidad de las mismas en nuestro medio. Por ello nos planteamos validar el modelo predictivo DASH y el nomograma de Viena a 12 meses.
MétodosEstudio retrospectivo de pacientes consecutivos no seleccionados con ETV no provocada desde 2006 hasta 2014. Comparamos la capacidad de predecir recurrencias de ETV de la escala DASH y el nomograma de Viena. La validación se realizó estratificando a los pacientes como de bajo o alto riesgo, según cada escala (discriminación) y comparando las recurrencias observadas frente a las esperadas (calibración).
ResultadosDe 353 pacientes evaluados, se analizaron 195, con una edad media de 53,5+/-19 años. Hubo 21 recurrencias a 1 año (10,8%, IC95%: 6,8-16%). Según la escala DASH, fueron catalogados de bajo riesgo el 42%, observando ETV recurrente en el grupo de bajo fue del 4,9% (IC95%: 1,3-12%) vs el grupo de alto riesgo en que fue del 15% (IC95%: 9-23%) (p<0,05). Según el nomograma de Viena, fueron catalogados de bajo riesgo el 30%, observando ETV recurrente en el grupo de bajo vs alto riesgo en el 4,2% (IC95%: 0,5-14%) vs 16,2% (IC95%: 9,9-24,4%) (p<0,05).
ConclusionesNuestro estudio valida la escala DASH y el nomograma de Viena en nuestra población. El modelo predictivo DASH sería el más aconsejable, tanto por su sencillez como por la capacidad de identificar a más pacientes de bajo riesgo frente al nomograma de Viena (42% vs 30%).
Venous thromboembolic disease (VTD) refers to both deep venous thrombosis (DVT) and pulmonary embolism (PE). VTD occurs at an incidence of 1/1000 individuals/year, and is the third most common cardiovascular disease, after acute coronary syndrome and stroke.1
VTD can have different underlying causes, and the pathophysiological mechanism involves a number of risk factors associated with the individual predisposition, be it genetic or acquired, of each subject. When these risk factors and predisposing conditions are known (e.g. surgery, immobilization, hormone treatment, pregnancy, puerperium, and cancer), VTD is said to be provoked. Even so, approximately 50% of VTDs occur in the absence of a clear precipitating factor, in which case they are classified as unprovoked or idiopathic.2–4
The optimal duration of anticoagulant treatment in patients with unprovoked VTD has not yet been determined, since the cumulative risk of recurrent VTD at 5 years in patients who discontinue anticoagulation is 25%–30%.4–6 For this reason, continued anticoagulant treatment is recommended if the risk of bleeding is low.4–7 However, current evidence points to risk factors, such as sex and D-dimer, that can modulate the risk of recurrence following discontinuation of anticoagulation.5,6,8 Practically speaking, we must analyze the case fatality rate of major bleeding, which is 2–3 times higher than the fatality rate of recurrent VTD,9,10 for which reason discontinuation of extended anticoagulant therapy is recommended when the risk of recurrence during the first year is less than 5%–8%.11–13
Several clinical prediction models3,14 have been developed and validated to individually assess the risk of recurrent VTD in patients with unprovoked VTD who discontinue anticoagulant treatment, although none has been validated in Spain. Two of the most widely known validated scales are the DASH prediction score13,15 and the Venice nomogram.16,17 The aim of this study was to perform an external validation in our setting of the DASH score and the Vienna nomogram, reevaluating the calibration of the predictions (e.g. the precision of the probability of recurrent VTD as predicted by the models compared with those observed in the new cohort).
MethodsStudy Design and Patient SelectionThis was a retrospective study of unselected, consecutive VTD patients evaluated in a dedicated clinic, between 2006 and 2014.
Inclusion criteria: Patients with unprovoked VTD who received anticoagulant therapy for at least 3 months. Patients with unprovoked VTD who discontinued anticoagulation with D dimer determinations performed 21 days after the withdrawal of anticoagulant treatment.
Exclusion criteria: Patients with provoked VTD meeting ISTH criteria2 (active cancer, surgery, immobilization, trauma, prior hospitalization, pregnancy, or puerperium; positive for anticardiolipin antibody or lupus anticoagulant).
The definitive diagnosis of DVT was established by Doppler or compressive ultrasound or venography.18 One of the following was required to confirm the diagnosis of PE: (1) intraluminal filling defect on contrast-enhanced spiral computed tomography (CT) scan of the chest; (2) high probability on ventilation-perfusion lung scintigraphy according to PIOPED criteria; or (3) ventilation-perfusion lung scintigraphy not diagnostic, with DVT of the lower limb confirmed by Doppler or compressive ultrasound.19–21
Data Collection and MonitoringWe collected a series of clinical variables from all patients, including at least those needed to validate the 2 scales: age, sex, comorbidities (including arterial hypertension, diabetes, dyslipidemia, ischemic heart disease), site of the thrombotic event, date of the thrombotic event, and discontinuation of anticoagulation therapy.
Prior to discontinuation, all patients had received anticoagulant treatment for at least 3 months, as indicated in clinical practice guidelines.6,22,23 D dimer was determined 21±5 days after discontinuation of anticoagulant treatment, and patients were followed up for at least 12 months after discontinuation of treatment, either in the clinic or by telephone. If the patient did not attend the visit, they were contacted by telephone, and administrative data were used if necessary to confirm patient death or absence of VTD (the digital clinical histories of the hospital and primary care were consulted). Peripheral blood was obtained by venipuncture of the antecubital fossa 21 days after discontinuing anticoagulant treatment. D dimer was determined using the Acute Care™ D dimer test pack, Siemens Healthcare Diagnostics (Newark, DE, US), the normal range of which is <500μg/L.
Patients with clinical suspicion of recurrent VTD were evaluated using the same method used for the initial diagnosis. Patients with clinical suspicion of recurrent VTD were evaluated using the same method used for the initial diagnosis. Patients with clinical suspicion of recurrent PE were documented by ventilation-perfusion scintigraphy, contrast-enhanced computed tomography, or pulmonary angiography. A diagnosis of recurrent PE was given if a high probability lung scintigraphy revealed a new area of segmental perfusion defect with ventilation changes, or if new filling defects were observed on the spiral CT. Patients with a clinical suspicion of recurrent DVT were evaluated by Doppler or compressive ultrasound, and the diagnosis of recurrent DVT required clear evidence of thrombosis in at least 1 segment of the proximal vein not previously affected. This required ultrasound evidence of a lack of compressibility in the pathway of previously compressible proximal veins. All examinations were carried out by experts in the technique used. An adjudication committee (LJP and SMR) reviewed and confirmed all recurrences.
The primary study objective was the external validation at 12 months of the DASH prediction score and the Vienna nomogram for VTD. Both calibration and discrimination were validated.24–26 Secondary objectives were: (1) comparative analysis of discrimination of the 2 scales; (2) long-term external validation of the 2 scales. The study followed the principles of ethical practice of the Declaration of Helsinki and was approved by the hospital ethics committee.
The DASH score evaluates elevated D dimer after discontinuation of anticoagulation (+2 points), age ≤50 years (+1 point), male sex (+1 point), and hormone treatment (−2 points). Patients with a score ≤1 or ≥2 are considered to be at low or high risk of recurrent VTD, respectively.15 This model was derived from a cohort of 1818 patients15 and subsequently validated in a retrospective cohort of 827 patients.13 The Vienna nomogram variables are sex, site of VTD (PE, proximal or distal DVT), and D dimer following discontinuation of anticoagulant treatment. This model was derived from a cohort of 925 patients16 and subsequently validated in a study of 904 patients using data from 7 studies.17 The Vienna nomogram score must be calculated for each patient, and then the sample is divided into 4 quartiles (Q). Patients in Q1 and Q2–4, are defined as low and high risk, respectively.16
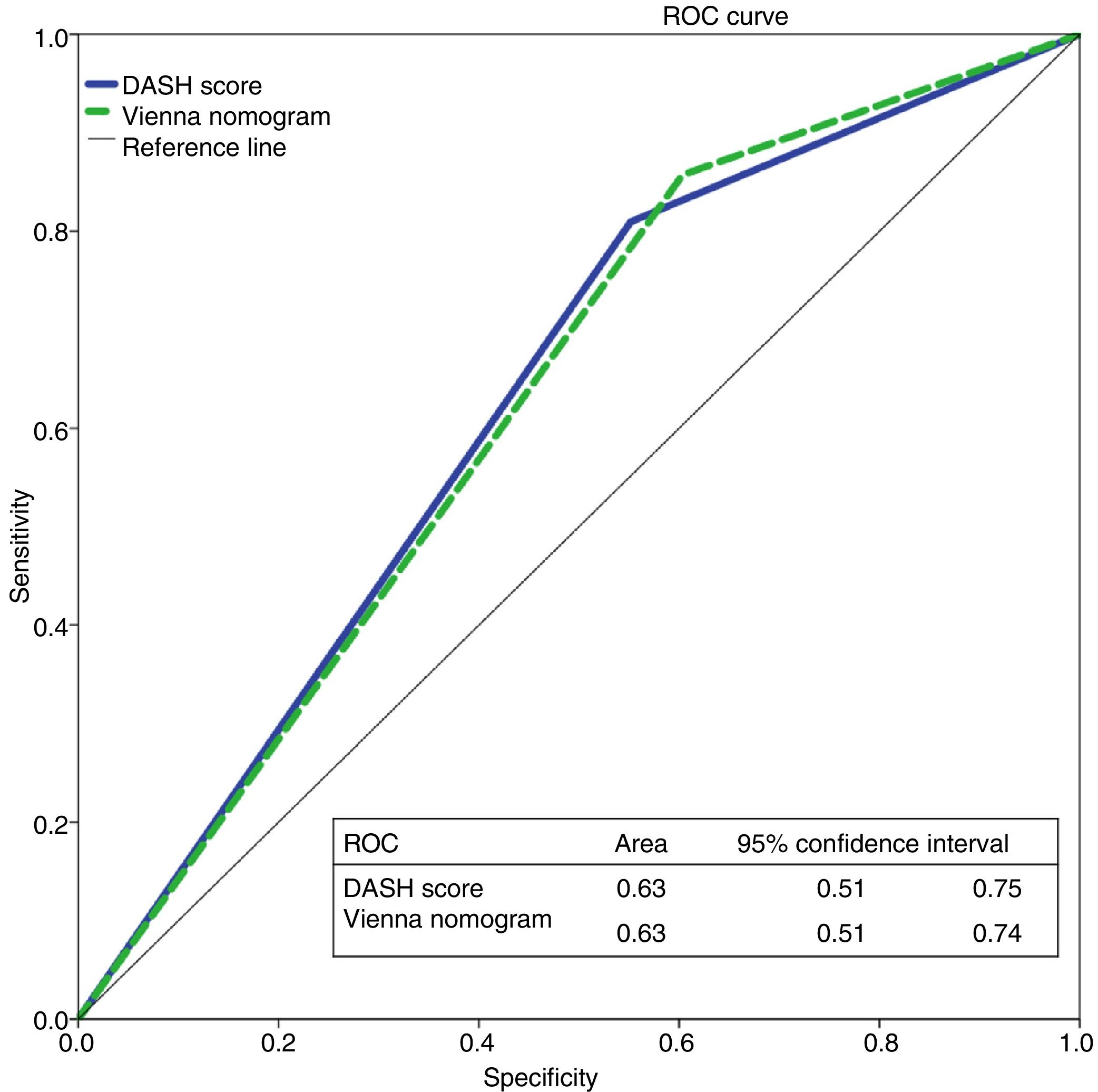
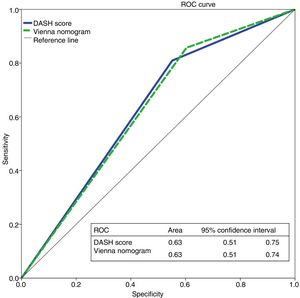
Statistical AnalysisContinuous variables were expressed as mean and standard deviation (SD) and categorical variables as frequencies and percentages. Both models were validated externally for calibration and discrimination.24–26 The calibration was performed by comparing observed events with expected events. The predicted data were retrieved from previous publications on the development of the DASH prediction score15 and the Vienna16 nomogram. The discrimination of the models was evaluated according to the degree to which the model distinguishes between individuals who have recurrent VTD compared to those who do not. To this end, we analyzed the percentage of patients identified as low-risk and used the area under the ROC curve. We analyzed confidence intervals using the Clopper–Pearson exact method and time to recurrence using the Kaplan–Meier method (Mantel–Cox Log Rank test). Statistical significance was set at P<.05. Statistical analyses were carried out using the SPSS v20 statistical package.
Sample Size CalculationAs suggested by the panel of experts, we calculated a sample size large enough to ensure that the risk of recurrent VTD in the low-risk group would be <5% per year, in order to demonstrate that patients with unprovoked VTD could safely discontinue their anticoagulant treatment.12 To identify significant differences, we assumed a rate of recurrent VTD at 1 year of 5% in the low-risk group and 14% in the high-risk group. For a 95% confidence level (1−α) with a statistical power of 80%, the minimum sample size was calculated to be 165 patients.
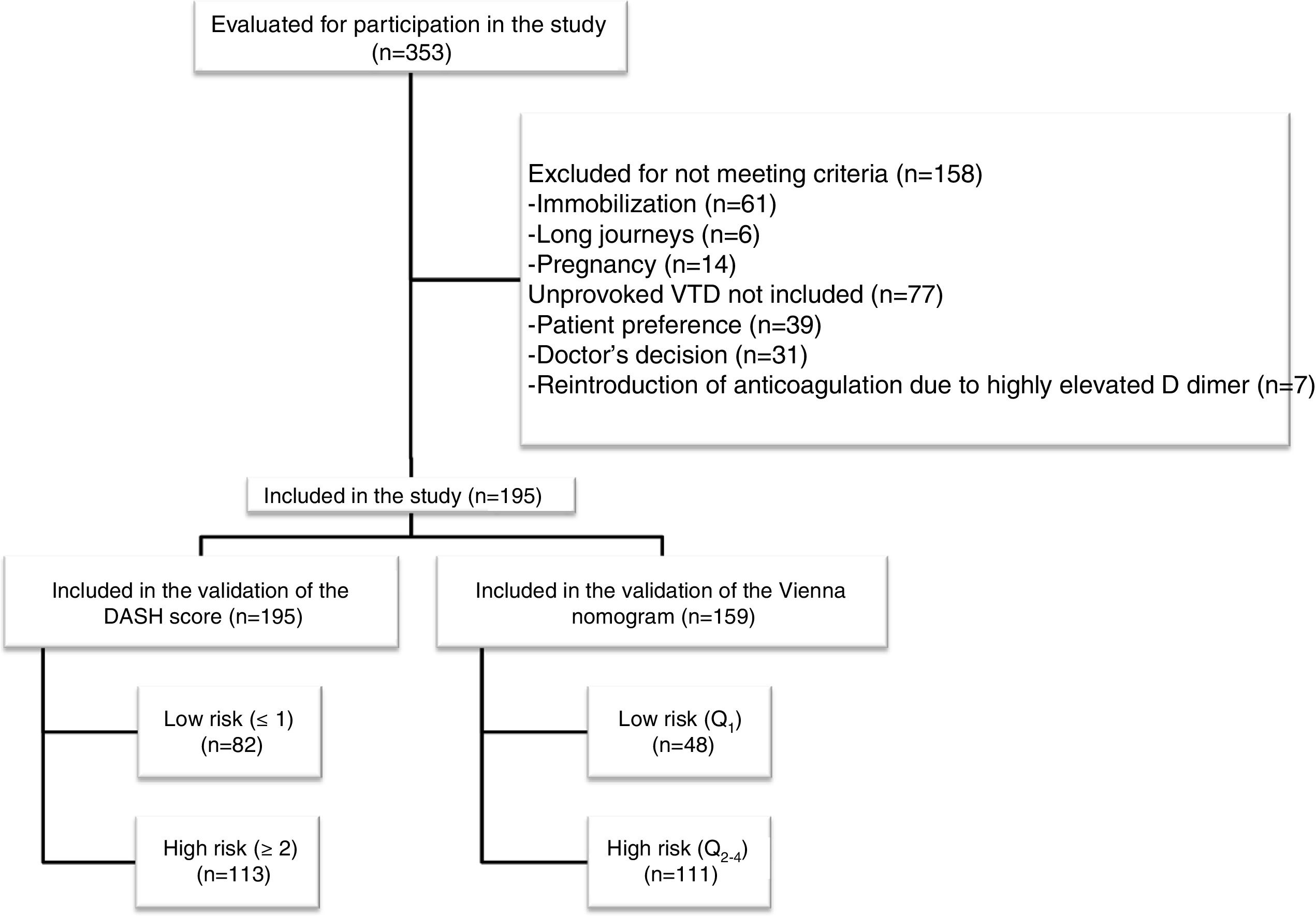
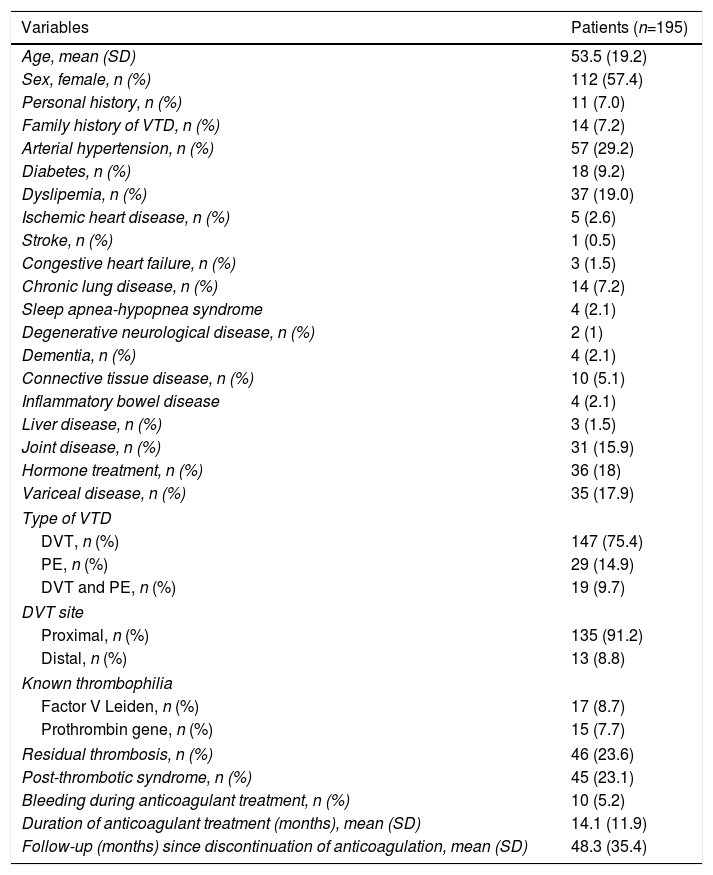
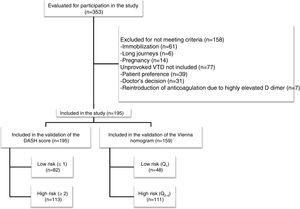
ResultsDuring the study period, we assessed 353 patients, of whom 195 met the inclusion criteria (flow chart, Fig. 1). All patients were Caucasian, with a mean age (±SD) of 53.5 (±19) years and a predominance of women (57%). VTD presented as: DVT (75.4%), PE (14.9%), and DVT plus PE (9.7%). A family history of VTD was recorded in 7.2% (n=14) of the patients, and 7% (n=11) had prior VTD. Of the 11 patients with a personal history of VTD, 80% (n=9) had previously had provoked VTD, and 20% (n=2) unprovoked VTD but with a high risk of bleeding for whom discontinuation of anticoagulant treatment was proposed. Table 1 shows the main characteristics of the study patients.
Study Patient Characteristics.
| Variables | Patients (n=195) |
|---|---|
| Age, mean (SD) | 53.5 (19.2) |
| Sex, female, n (%) | 112 (57.4) |
| Personal history, n (%) | 11 (7.0) |
| Family history of VTD, n (%) | 14 (7.2) |
| Arterial hypertension, n (%) | 57 (29.2) |
| Diabetes, n (%) | 18 (9.2) |
| Dyslipemia, n (%) | 37 (19.0) |
| Ischemic heart disease, n (%) | 5 (2.6) |
| Stroke, n (%) | 1 (0.5) |
| Congestive heart failure, n (%) | 3 (1.5) |
| Chronic lung disease, n (%) | 14 (7.2) |
| Sleep apnea-hypopnea syndrome | 4 (2.1) |
| Degenerative neurological disease, n (%) | 2 (1) |
| Dementia, n (%) | 4 (2.1) |
| Connective tissue disease, n (%) | 10 (5.1) |
| Inflammatory bowel disease | 4 (2.1) |
| Liver disease, n (%) | 3 (1.5) |
| Joint disease, n (%) | 31 (15.9) |
| Hormone treatment, n (%) | 36 (18) |
| Variceal disease, n (%) | 35 (17.9) |
| Type of VTD | |
| DVT, n (%) | 147 (75.4) |
| PE, n (%) | 29 (14.9) |
| DVT and PE, n (%) | 19 (9.7) |
| DVT site | |
| Proximal, n (%) | 135 (91.2) |
| Distal, n (%) | 13 (8.8) |
| Known thrombophilia | |
| Factor V Leiden, n (%) | 17 (8.7) |
| Prothrombin gene, n (%) | 15 (7.7) |
| Residual thrombosis, n (%) | 46 (23.6) |
| Post-thrombotic syndrome, n (%) | 45 (23.1) |
| Bleeding during anticoagulant treatment, n (%) | 10 (5.2) |
| Duration of anticoagulant treatment (months), mean (SD) | 14.1 (11.9) |
| Follow-up (months) since discontinuation of anticoagulation, mean (SD) | 48.3 (35.4) |
DVT: deep vein thrombosis; PE: pulmonary embolism; SD: standard deviation; VTD: venous thromboembolic disease.
Bleeding was the reason for proposing the discontinuation of anticoagulant treatment in 5.2%. Mean time (±) receiving anticoagulation and mean follow-up after discontinuing anticoagulant treatment was 14.1 (±11.9) and 48.3 (±35.4) months, respectively.
We evaluated the 195 patients using the DASH score, and 42% and 58% of patients were classified as high and low risk, respectively. To apply the Vienna nomogram, we excluded patients receiving hormone treatment (n=36), giving a final sample of 159 patients who were classified as low vs high risk, 30% and 70%, respectively. Fig. 2 shows the ROC curve evaluating the probability of each of the scales predicting recurrent VTD at 12 months.
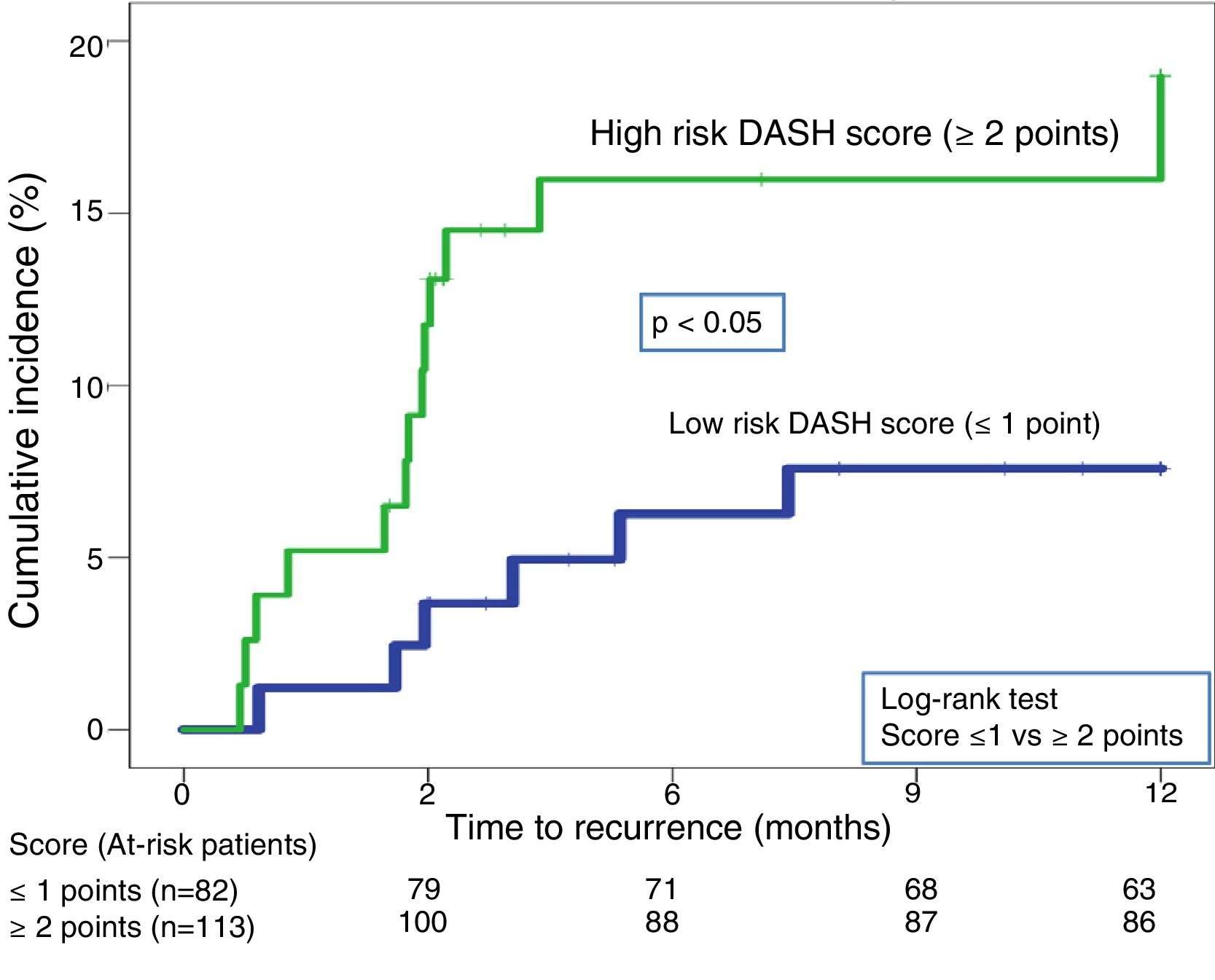
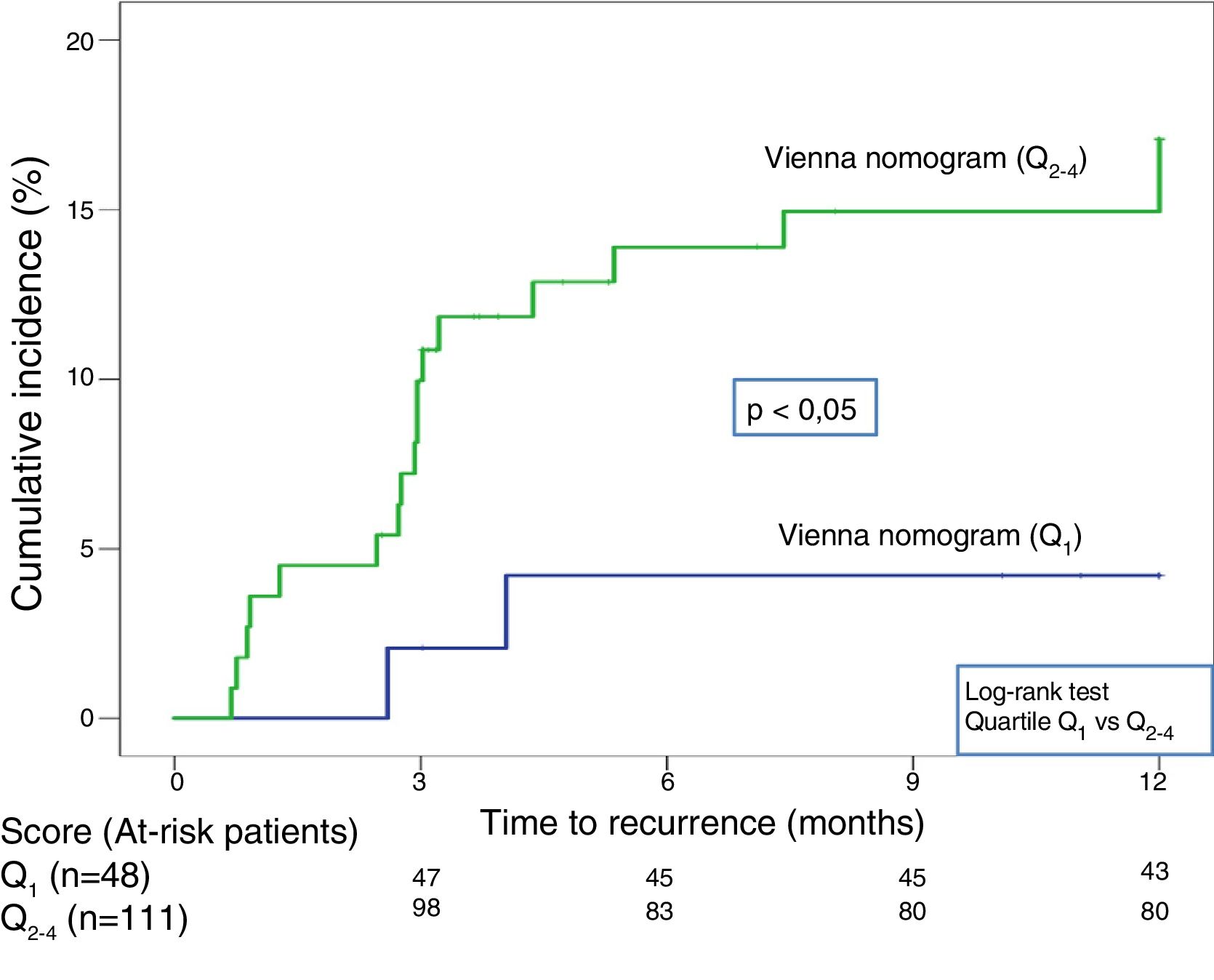
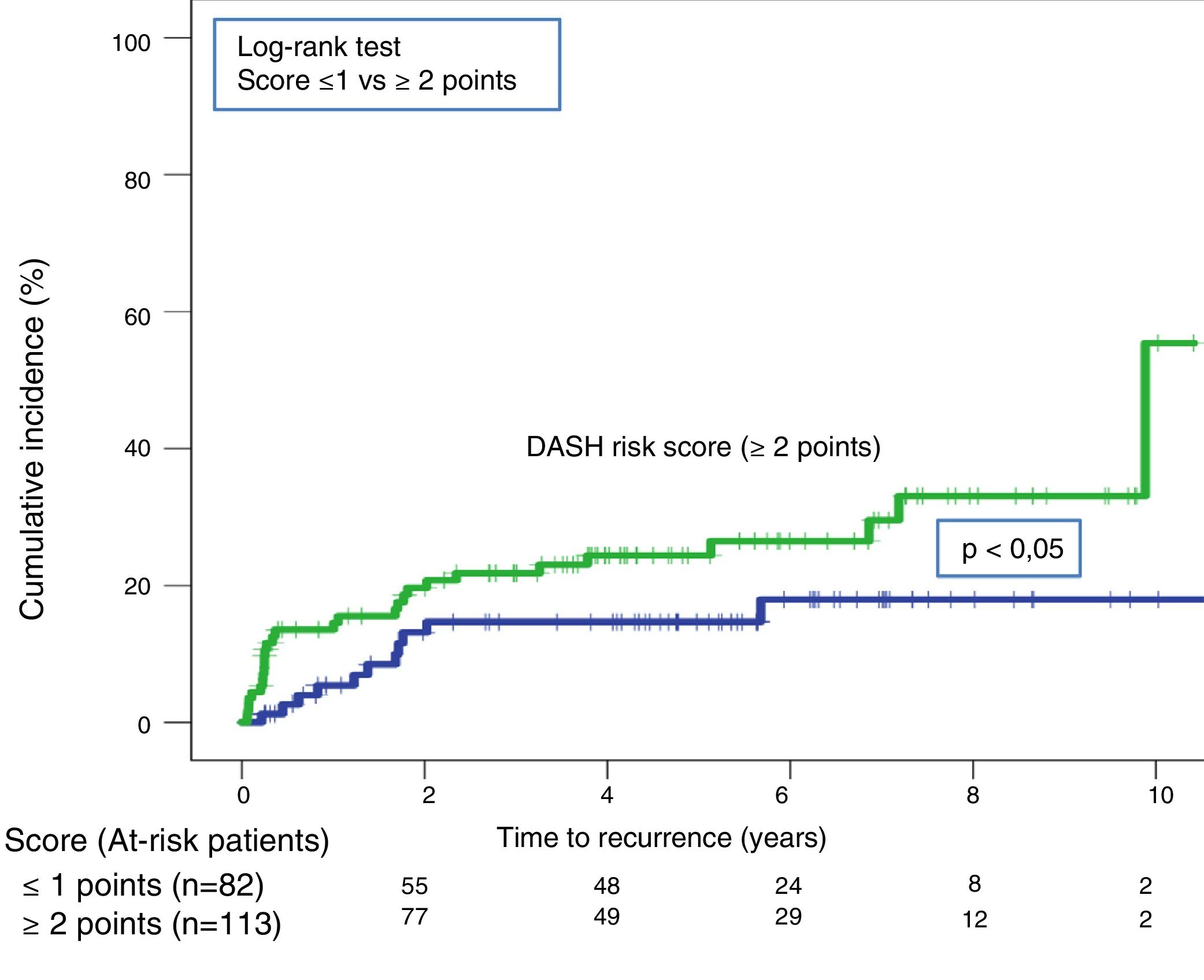
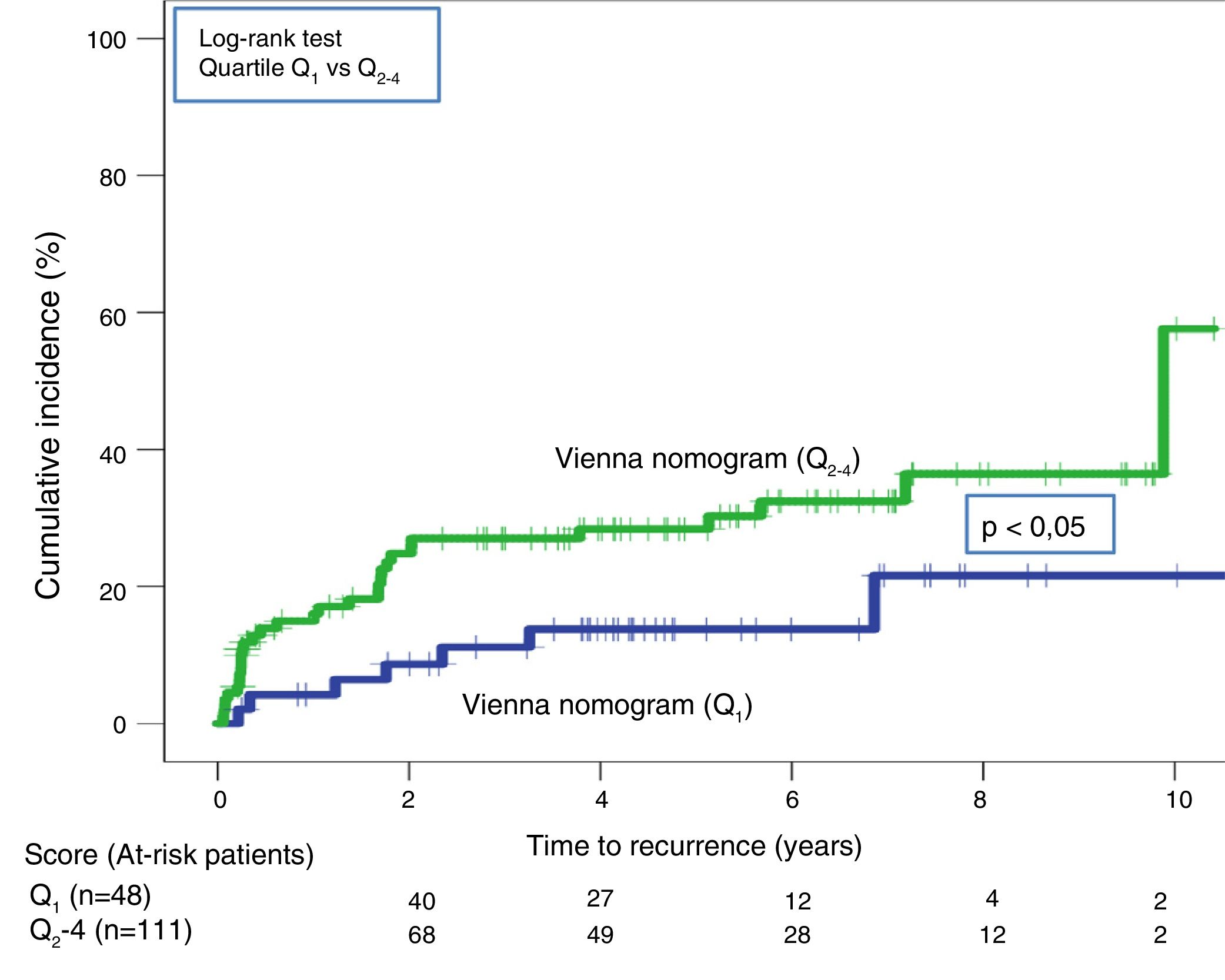
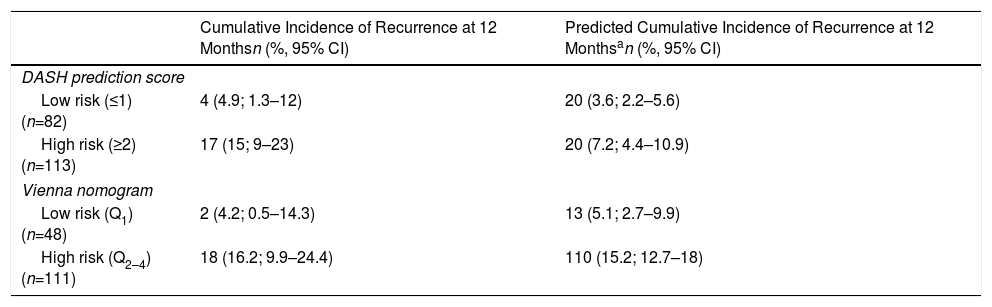
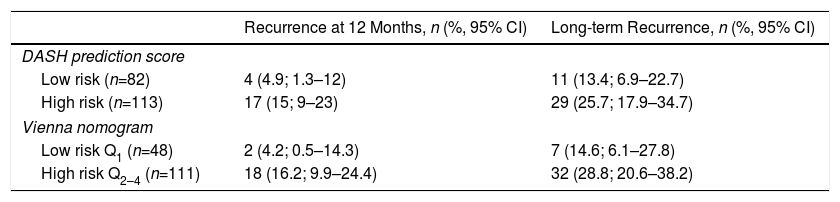
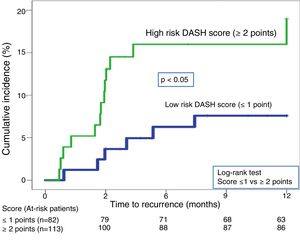
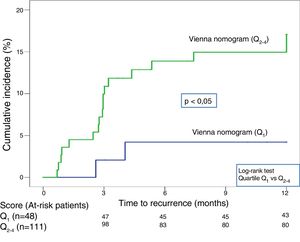
Twenty-one recurrences were observed in the validation of the DASH score (n=195) (10.8%, 95% CI: 6.8%–16%) during the first 12 months after anticoagulant treatment was discontinued. In 95% of cases (n=20), recurrences presented as DVT. The cumulative incidence in low and high-risk patients was 4.9% (95% CI: 1.3%–12%), and 15% (95% CI: 9%–23%), respectively (P<.05) (Fig. 3). Twenty recurrences were observed in the validation of the Vienna nomogram (n=159) (12.6%, 95% CI: 7.9%–18.8%) during the first 12 months after anticoagulant treatment was discontinued. After completing the Vienna nomogram, we divided the sample by quartiles (Q), observing a cumulative incidence of recurrence of 4.2% (95% CI: 0.5%–14%) in Q1 vs. 16.2% (95% CI: 9.9%–24.4%) in Q2–4 (P<.05) (Fig. 4). We calibrated both models by comparing the observed incidence of recurrent VTD with the incidence predicted in the validation cohorts (Table 2).
Calibration of the Validation. Baseline Characteristics Compared to Initial Cohort.
| Cumulative Incidence of Recurrence at 12 Monthsn (%, 95% CI) | Predicted Cumulative Incidence of Recurrence at 12 Monthsan (%, 95% CI) | |
|---|---|---|
| DASH prediction score | ||
| Low risk (≤1) (n=82) | 4 (4.9; 1.3–12) | 20 (3.6; 2.2–5.6) |
| High risk (≥2) (n=113) | 17 (15; 9–23) | 20 (7.2; 4.4–10.9) |
| Vienna nomogram | ||
| Low risk (Q1) (n=48) | 2 (4.2; 0.5–14.3) | 13 (5.1; 2.7–9.9) |
| High risk (Q2–4) (n=111) | 18 (16.2; 9.9–24.4) | 110 (15.2; 12.7–18) |
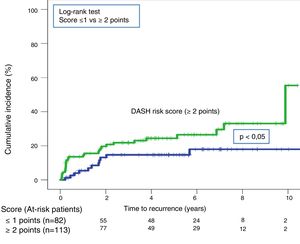
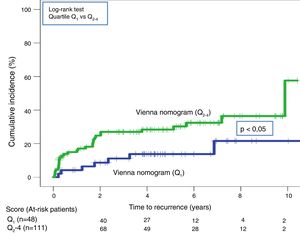
Forty recurrences (20.5%; 95% CI: 15.1–26.9) occurred during follow-up (median 3.9 years; interquartile range: 1.1–6.3 years). After application of the DASH score, recurrences in low-risk and high-risk patients were 13.4% (95%; 6.9%–22,7%) and 25.7% (95% CI: 17.9%–34.7%), respectively (P=.05) (Table 3, Fig. 5). For the Vienna nomogram, the cumulative incidence in low-risk and high-risk patients was 14.6% (95% CI: 6.1%–27.8%) and 28.8% (95% CI: 20.6%–38.2%), respectively (P<.05) (Fig. 6).
Recurrence in Patients Stratified According to DASH Score and VIENNA Nomogram.
| Recurrence at 12 Months, n (%, 95% CI) | Long-term Recurrence, n (%, 95% CI) | |
|---|---|---|
| DASH prediction score | ||
| Low risk (n=82) | 4 (4.9; 1.3–12) | 11 (13.4; 6.9–22.7) |
| High risk (n=113) | 17 (15; 9–23) | 29 (25.7; 17.9–34.7) |
| Vienna nomogram | ||
| Low risk Q1 (n=48) | 2 (4.2; 0.5–14.3) | 7 (14.6; 6.1–27.8) |
| High risk Q2–4 (n=111) | 18 (16.2; 9.9–24.4) | 32 (28.8; 20.6–38.2) |
We performed a retrospective validation of the use of the DASH prediction score and the Vienna nomogram in our setting. These scales are used to identify patients with unprovoked VTD and a low risk of recurrence, in order to safely discontinue anticoagulant treatment indefinitely after at least 3 months of treatment. The implications of these results are that the duration of anticoagulant treatment may be optimized by safely discontinuing it in patients classified as low risk of recurrence, as shown by previous studies.11–13
The discontinuation of anticoagulant treatment in patients with unprovoked VTD is a hotly debated and controversial topic.12,27–29 In patients with unprovoked VTD, the guidelines recommend indefinite anticoagulant treatment unless the risk of bleeding is high.6,30 The International Society of Thrombosis and Hemostasis (ISTH) suggests that the discontinuation of anticoagulant treatment is appropriate in subgroups of patients with less than 5% risk of recurrent VTD at 1 year.12 Our study has validated both scores in a population with an incidence in the low-risk group of less than 5%. Given that the DASH score classifies more patients as low-risk than the Vienna nomogram (42% vs. 30%) and that it is easier to use, this would be the tool of choice.
Our study has several strengths. First, both scores are analyzed in the same patient cohort, so they can not only be validated, but also compared to see which includes more patients in the low-risk group, that is to say, the group that will benefit from the safe discontinuation of anticoagulation. To our knowledge, this is the only study that has validated these scales in Spain, giving them external validity and justifying their use in our setting (known as portability). Second, the clinical characteristics of our patients are similar to those of other previously published series. In our population the mean age was 54±19 years; in other studies, age ranged from 54 to 68 years, depending on the study.13,15–17 Third, all recurrences were documented objectively and reviewed by an independent committee (SMR and LJP).
Our study has some limitations. First, it is a retrospective study, with the inherent limitations of this design. However, it analyzes unselected consecutive patients with VTD. The DASH score and Vienna nomogram were also validated in retrospective patient series.13,17 It must be borne in mind that in some cases there may have been a selection bias, since in patients with a high risk of recurrent VTD, the clinician did not discontinue anticoagulation, although this limitation is also a feature of other previously published studies, due to their retrospective nature.13,17 In our study, we documented the number of patients who chose to continue anticoagulant treatment (14%), those in whom the clinician decided to maintain treatment (11%), and those in whom treatment was reintroduced after the detection of very high D dimer levels (3%). Similarly, in the validation study of the Vienna model, only 904 patients from a database of 1818 (50%) could be included in the external validation of the model.17 In the external validation of the DASH prediction score, no mention is made and no information is provided regarding patients who were not analyzed or who were excluded.13 Although it is preferable to perform the external validation prospectively from a methodological perspective, from a practical point of view we wished to determine the applicability and validity of both scales in our environment. Second, we have not analyzed other models, such as the Men-HERDOO2 rule,31 which evaluates sex (all men continue to receive anticoagulation), hyperpigmentation, edema or redness in the affected leg, D dimer during anticoagulation, and age. Although this method was validated prospectively, it has limitations, including the subjective assessment of signs in the affected limb and the fact that all men must continue anticoagulation indefinitely, parameters that do not figure in the other 2 scales. We could not validate this scale in our setting, as we lacked data on hyperpigmentation, edema or redness in the affected leg. Third, all our patients were Caucasian, which potentially limits generalization to other races. Fourth, we do not know the potential role of direct-acting oral anticoagulants when considering discontinuing anticoagulants, as these drugs have demonstrated a good safety profile, to the extent that some experts argue that if the annual risk of bleeding is greater than 3%, treatment should be discontinued, and if it is less than 3% a year, discontinuation should be considered if the patient is at low risk of recurrent VTD after evaluating their score.28 Nevertheless, this approach needs to be evaluated in a clinical trial. Fifth, it would have been interesting to conduct a subgroup analysis on the basis of gender or VTD site. Unfortunately, the sample was calculated to validate both scales, and our study does not have enough power to perform subanalyses.
ConclusionOur study validates the DASH score and the Vienna nomogram in our population. The DASH prediction score may be the most useful, because of both its simplicity and its ability to identify more low-risk patients than the Vienna nomogram (42% vs. 30%).
Conflict of InterestsLuis Jara Palomares has received honoraria for speaking engagements and travel and accommodation expenses from Rovi, Pfizer, Menarini, Leo-Pharma and GSK, unrelated to this manuscript. Maria Isabel Asensio Cruz has received travel and accommodation expenses from Rovi, Novartis, and Teva, unrelated to this manuscript. Raquel Morillo Guerrero has received honorariafor speaking engagements and travel and accommodation expenses from Pfizer, Menarini, and GSK, unrelated to this manuscript. The other authors state that they have no conflict of interests.
Please cite this article as: Marín-Romero S, Elías-Hernández T, Asensio-Cruz MI, Ortega-Rivera R, Morillo-Guerrero R, Toral J, et al. Riesgo de recurrencia tras retirada de la anticoagulación en pacientes con enfermedad tromboembólica venosa no provocada: validación externa del nomograma de Viena y del modelo predictivo DASH. Arch Bronconeumol. 2019;55:619–626.