There is limited knowledge on the relationship between lower respiratory tract infections (LRTI) and asthma and wheezing during infancy, as there are few studies with prospective design, birth cohort and in non selected population. The objectives of the present study were to determine the prevalence of asthma and recurrent wheezing in childhood and to analyse the relationship between LTRI during the first year of life and the development of asthma and/or wheezing in childhood.
Patients and MethodsProspective birth cohort study conducted in the Hospital del Mar (Barcelona, Spain). We recruited 487 children, followed up from the pregnancy to the 6th year of life. As outcomes we studied: the presence of asthma and wheezing. As independent variables we studied: LTRI occurring during the first year of life, and some covariables including, among others: prematurity, birth weight, maternal history of asthma and atopy, breastfeeding, prenatal exposure to tobacco.
ResultsThe asthma prevalence at 6 years of age was 9.3%. The variables associated with the development of asthma were LTRI, prematurity, atopic mother and formula breastfeeding. LTRI during the first year of life were also related with early recurrent wheezing and persistent wheezing.
ConclusionsOur results confirm that LTRI during the first year of life are related to the diagnosis of asthma and with the clinical phenotypes of early wheezing and persistent wheezing. These results are in accordance with the concept that LTRI occurring during a critical period of development, as are the first years of life, have an important role in the later development of asthma and recurrent wheezing.
En la relación entre las infecciones respiratorias de vías bajas (IRVB) y el desarrollo de asma y sibilancias durante la infancia, existen pocos datos con diseños prospectivos, de cohorte, desde el nacimiento y con población no seleccionada. El objetivo es determinar la prevalencia de asma y sibilancias recurrentes en la infancia, y establecer el efecto de las IRVB durante el primer año de vida
Pacientes y métodosCohorte poblacional de 487 recién nacidos en el Hospital del Mar, Barcelona, con seguimiento hasta los 6 años de edad. Como variables dependientes se han estudiado: presencia de asma y sibilancias; como variables independientes: IRVB ocurridas en el primer año de vida y diversas covariables, como prematuridad, peso al nacer, antecedentes maternos de asma y atopia, lactancia materna y exposición prenatal al tabaco.
ResultadosLa prevalencia de asma a los 6 años fue del 9,3%. Las variables asociadas al desarrollo de asma son: IRVB, ser prematuro, tener madre atópica y haber tomado lactancia artificial. Las IRVB en el primer año de vida también son un factor de riesgo relacionadas con las sibilancias recurrentes precoces y las sibi-lancias persistentes.
ConclusionesLos resultados confirman que las IRVB durante el primer año de vida están relacionadas con el diagnóstico de asma y con los fenotipos clínicos de sibilancias precoces y de sibilancias persistentes. Estos resultados concuerdan con el concepto de que las IRVB producidas en un período crítico del desarrollo, como los primeros años de vida, tienen un papel importante en la aparición posterior de asma y de sibilancias recurrentes.
Asthma is the most common chronic disease among children and its prevalence has increased in recent years,1 although the reasons for this increase are not conclusive. Regarding asthma in children, it is also important to bear in mind associated morbidity, the healthcare, social and family burden, and its possible relationship with chronic respiratory diseases in adulthood.
The study of asthma is extremely complex. Giving the right clinical definition of asthma is not easy as it can manifest itself at any age, with a wide range of phenotypes, with mild or severe crises, and also with remissions and exacerbations. Furthermore, wheezing is one of the main clinical manifestations of asthma, being common in children under 3, and generating considerable clinical heterogeneity. However, some groups of experts have grouped the onset of wheezing in childhood into a few phenotype categories: early, late-onset and persistent wheezing. According to these initial phenotype definitions by Martínez et al,2 asthma is not classified exclusively by age of onset, but rather depending on the appearance of wheezing during childhood.
Furthermore, its aetiology is multifactorial, different genes appearing to influence the function of the respiratory and immune systems, and in addition this genetic susceptibility can be affected by many environmental or lifestyle factors. Recent studies have shown that environmental factors, particularly those taking place during the first years of life, may play an important role in children's structural development or that of their immune system. On this subject, exposure to different factors and allergens, besides certain infections, could give rise to a higher likelihood of allergies or asthma.
Most respiratory infections during childhood affect the upper respiratory tract, although there is also a high incidence of lower respiratory tract infections (LRTI), such as bronchitis, laryngotracheobronchitis, bronchiolitis or pneumonia. In developed countries, between 20% and 39% of the child population have a LRTI.3,4 Epidemiological data show that respiratory infections are related to developing asthma later in life. For example, children with asthma had a mean of 2.5 episodes of LRTI per year, while others only had 1 episode, and also in around 70% of episodes of LRTI before 3 years, it is possible to demonstrate the presence of respiratory viruses.5 The temporal relationship between viral respiratory infections and respiratory processes accompanied by wheezing in children has also been established through microbiological and serum tests for the most common viruses (for example, RSV, rhinovirus, metapneumovirus, bocavirus, parainfluenza virus, and influenza virus).6–9 Other studies show that the incidence of asthma crises associated with viral infections is between 24% and 54%.10,11
However, in the study of the relationship between LRTIs and the development of asthma and wheezing during childhood, there is a lack of data from studies with a prospective design, specifically, birth cohort studies with a population which has not been selected because of a family history of asthma/atopy or treated in a hospital setting. Identifying risk factors in the general population for having asthma during childhood and its relationship with the clinical phenotypes described may make it possible to improve both the treatment of acute clinical symptoms and the use of long-term pharmacological and environmental preventative measures.
The aim of this study is to determine the prevalence of asthma and wheezing in children in Spain. Furthermore, this article studies the influence of LRTIs during the first year of life as a risk factor for developing asthma and wheezing throughout childhood.
MethodsFour hundred and eighty seven children were selected from the influence area of the Hospital del Mar (Barcelona, Spain) between 1996 and 1998, and who were monitored from birth until 6 years of age. This group of children formed part of one of the cohorts included in the Asthma Multicenter Cohort Study (AMICS) which included 4 different geographical locations: Ashford (United Kingdom), Barcelona and Menorca (Spain) and Munich (Germany). The follow-up cohort is the same as that described previously.12 The study was approved by the Ethics Committee for Medical Research at the hospitals and the parents gave their written, informed consent.
Asthma and WheezingThe diagnosis of asthma was established clinically from a positive response to the question, “Has a doctor ever said that your child has asthma?” The diagnosis of wheezing was established clinically from a positive response to the question, “Has a doctor ever said that your child has a wheezy chest?” This information was obtained annually using validated questionnaires, which included questions from the International Study of Asthma and Allergy in Children (ISAAC).13 Each child was assigned a wheezing phenotype, following those proposed by Martinez et al2: never had wheezing, early and transitory wheezing (the first episode began during first year and no new episodes after 3 years), late-onset wheezing (first episode began after 3 years), and persistent wheezing (onset before 3 years and continues at 6 years of age).
Lower Respiratory Tract InfectionsActive monitoring of LRTIs was performed during the first year of life, following this action protocol: the day after giving birth the parents were informed about infections of the lower respiratory tract and the symptoms they had to inform of if they identified them in their child; they were given a contact telephone number with a 24 h answering machine; the research staff contacted the parents to carry out a home visit between 24 and 48 h after the onset of symptoms; the call was registered as “yes LRTI” or “no LRTI” depending on whether the LRTI criteria were met; at home, the child was given a standard medical examination, the diagnosis was confirmed, a questionnaire was completed about respiratory morbidities, and a nasopharyngeal mucus sample was taken. Furthermore, to avoid the loss of respiratory illnesses of interest, a monthly telephone call was made to the family to check that the child had not had symptoms in the previous few days and to reinforce the action protocol.
The LRTIs diagnosed in accordance with the clinical characteristics defined by Denny and Clyde14 were laryngotracheobronchitis, bronchitis, bronchiolitis and pneumonia, as well as non-specific LRTIs when the infection could not be classified into any of the previous categories.
The techniques for collecting the nasopharyngeal sample and for identifying the virus, and the results obtained from the samples are detailed extensively in a previous publication.15
CovariablesThe variables obtained from the questionnaire were:
Personal: sex of the new born; prematurity (<37 weeks gestation); weight at birth (<2,500, 2,500–4,000 and >4,000 grams); length at birth (<50 and >50 cm); small for gestational age (
Family: mother's age; mother's country of origin; socio-economic level (defined by father's occupation and in accordance with the classification of the UK Registrar General's 1990); maternal or paternal history of asthma; maternal history of atopy (when the mother states she has suffered from rhinitis or eczema); number of siblings.
Home environment: presence of pets at home in 1st and 4th year.
The assessments carried out were:
Personal: total immunoglobulin E (IgE) in cord serum (Pharmacia CAP System low range FEIA, Pharmacia, Freiburg, Germany); total IgE to Der p1 and Fel d1 in child's blood at 4 years of age (Pharmacia CAP System RAST-FEIA, Pharmacia, Freiburg, Germany); in both cases IgE was defined as high when levels were over 0.35 UI/ml; skin allergy tests for allergens at 6 years of age: Der p1, Der p2, Fel d1, dog hair, parietaria, gramineae, tree pollen and grass pollen (LETI Prick Test, LETI laboratories SL, Spain), the response was considered positive when the reaction to one of the allergens was over 3mm; maximum expiratory flow (MEF) was measured (MicroPeak, Micro Medical Ltd) before and after exercise, and the respiratory response to exercise was calculated as the variation in MEF>15% and>20% (after 16min) after exercise compared with the MEF prior to the start of the test.
Family: Specific IgE to Der p1 in maternal blood (Pharmacia CAP System RAST-FEIA, Pharmacia, Freiburg, Germany); IgE was defined as being high when levels were over 0.35 UI/ml.
Home environment: cotinine in cord serum and in the child's urine at 4 years with radioimmunoanalysis following the method described by Pichini et al16 (>14 ng/ml identifies newborns whose mother smoked during pregnancy); at 6 months old dust samples were analyzed which were collected from the child's cot and from the floor of the living room of their home to determine by ELISA the concentration of aeroallergens (Der p1 and Fel d1)17 and the exposure categories proposed by Platts-Mills and de Weck were used,18 also, at 6 months, a specific passive monitor (Toyo Roshi NO2 filter badges, Tokyo, Japan) was placed in the living room for 1 week to measure the concentration of NO2, following the method described by Yanagisawa and Nishimura.19
Statistical AnalysisThe prevalence of asthma and the wheezing phenotypes were determined in the 6th year of life, as well as wheezing frequency (never, occasional, always) in each year of monitoring. The Chi-squared test was used to compare prevalences between groups and the Mantel-Haenzel test to analyze the tendencies between categories. The odds ratio (OR) was calculated with a confidence interval of 95% (CI 95%), using a univariate logistic regression model to estimate the relationship of the variables with statistically significant differences. Multivariate logistic regression models were performed to assess the simultaneous effect of the variables studied (asthma diagnosis and wheezing phenotypes) and the risk factors found in the univariate analysis. When the variable under study was the asthma diagnosis at 6 years of age the forward stepwise approach was used (likelihood ratio), with entry of variables with P<.10 and exit with P>.20. When the study variable was one of the wheezing phenotypes, the method was multinomial. Variables with a value of P<.20 in the univariate analysis were included in the multivariate analyses. Statistical significance in these models was based on the likelihood ratio. All the analyses were performed with the SPSS statistics software (SPSS version 16.0, SPSS Inc, Chicago, IL, USA).
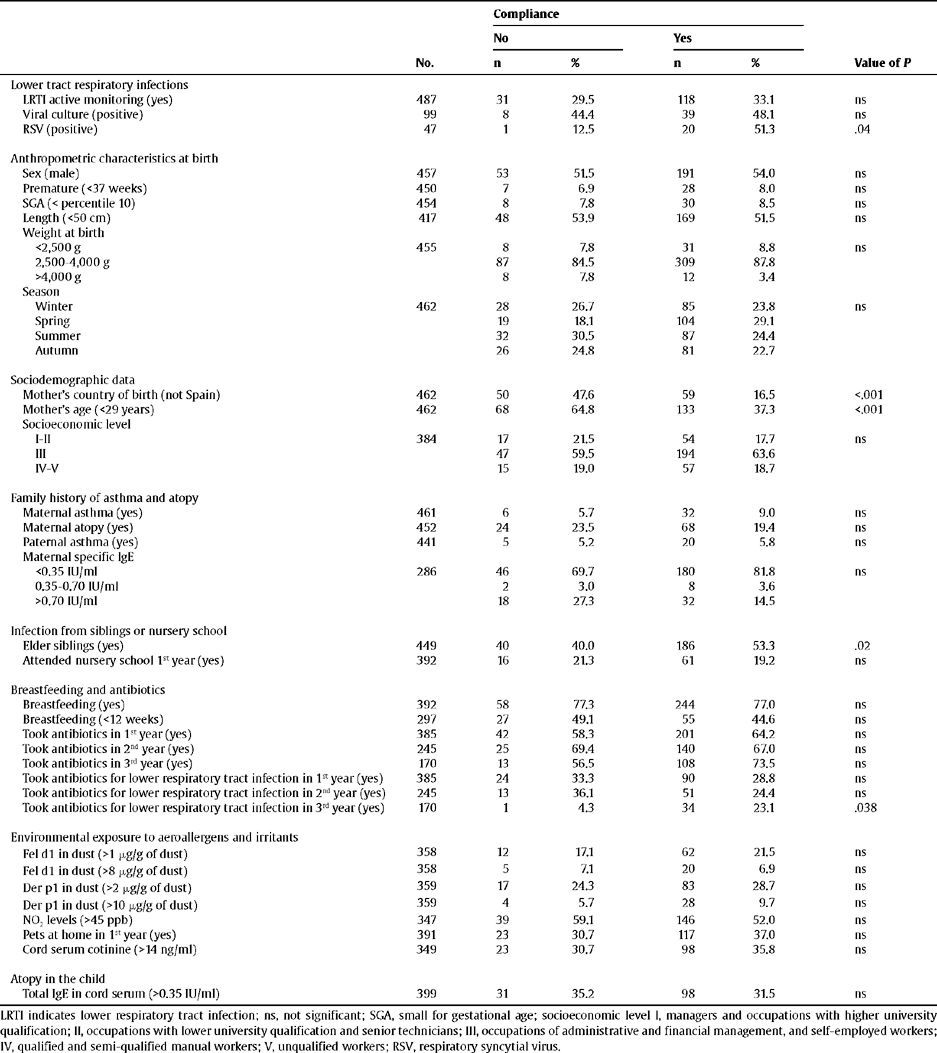
ResultsFollow-up of the CohortOf the 487 newborns who started the study, information was obtained until the end of the study for 368 (75.6%). The reasons for dropping out of the study were the lack of a current telephone number and moving home to outside of Barcelona. The children who did not finish the study had immigrant mothers (P<.001), mothers under 29 years of age (P<.001) and had no elder siblings (P=.02). Statistically significant differences were not found for the other risk factors (Table 1).
Monitoring data from the cohort (interview and biological samples)
| Compliance | ||||||
| No | Yes | |||||
| No. | n | % | n | % | Value of P | |
| Lower tract respiratory infections | ||||||
| LRTI active monitoring (yes) | 487 | 31 | 29.5 | 118 | 33.1 | ns |
| Viral culture (positive) | 99 | 8 | 44.4 | 39 | 48.1 | ns |
| RSV (positive) | 47 | 1 | 12.5 | 20 | 51.3 | .04 |
| Anthropometric characteristics at birth | ||||||
| Sex (male) | 457 | 53 | 51.5 | 191 | 54.0 | ns |
| Premature (<37 weeks) | 450 | 7 | 6.9 | 28 | 8.0 | ns |
| SGA (< percentile 10) | 454 | 8 | 7.8 | 30 | 8.5 | ns |
| Length (<50 cm) | 417 | 48 | 53.9 | 169 | 51.5 | ns |
| Weight at birth | ||||||
| <2,500 g | 455 | 8 | 7.8 | 31 | 8.8 | ns |
| 2,500–4,000 g | 87 | 84.5 | 309 | 87.8 | ||
| >4,000 g | 8 | 7.8 | 12 | 3.4 | ||
| Season | ||||||
| Winter | 462 | 28 | 26.7 | 85 | 23.8 | ns |
| Spring | 19 | 18.1 | 104 | 29.1 | ||
| Summer | 32 | 30.5 | 87 | 24.4 | ||
| Autumn | 26 | 24.8 | 81 | 22.7 | ||
| Sociodemographic data | ||||||
| Mother's country of birth (not Spain) | 462 | 50 | 47.6 | 59 | 16.5 | <.001 |
| Mother's age (<29 years) | 462 | 68 | 64.8 | 133 | 37.3 | <.001 |
| Socioeconomic level | ||||||
| I-II | 384 | 17 | 21.5 | 54 | 17.7 | ns |
| III | 47 | 59.5 | 194 | 63.6 | ||
| IV-V | 15 | 19.0 | 57 | 18.7 | ||
| Family history of asthma and atopy | ||||||
| Maternal asthma (yes) | 461 | 6 | 5.7 | 32 | 9.0 | ns |
| Maternal atopy (yes) | 452 | 24 | 23.5 | 68 | 19.4 | ns |
| Paternal asthma (yes) | 441 | 5 | 5.2 | 20 | 5.8 | ns |
| Maternal specific IgE | ||||||
| <0.35 IU/ml | 286 | 46 | 69.7 | 180 | 81.8 | ns |
| 0.35–0.70 IU/ml | 2 | 3.0 | 8 | 3.6 | ||
| >0.70 IU/ml | 18 | 27.3 | 32 | 14.5 | ||
| Infection from siblings or nursery school | ||||||
| Elder siblings (yes) | 449 | 40 | 40.0 | 186 | 53.3 | .02 |
| Attended nursery school 1st year (yes) | 392 | 16 | 21.3 | 61 | 19.2 | ns |
| Breastfeeding and antibiotics | ||||||
| Breastfeeding (yes) | 392 | 58 | 77.3 | 244 | 77.0 | ns |
| Breastfeeding (<12 weeks) | 297 | 27 | 49.1 | 55 | 44.6 | ns |
| Took antibiotics in 1st year (yes) | 385 | 42 | 58.3 | 201 | 64.2 | ns |
| Took antibiotics in 2nd year (yes) | 245 | 25 | 69.4 | 140 | 67.0 | ns |
| Took antibiotics in 3rd year (yes) | 170 | 13 | 56.5 | 108 | 73.5 | ns |
| Took antibiotics for lower respiratory tract infection in 1st year (yes) | 385 | 24 | 33.3 | 90 | 28.8 | ns |
| Took antibiotics for lower respiratory tract infection in 2nd year (yes) | 245 | 13 | 36.1 | 51 | 24.4 | ns |
| Took antibiotics for lower respiratory tract infection in 3rd year (yes) | 170 | 1 | 4.3 | 34 | 23.1 | .038 |
| Environmental exposure to aeroallergens and irritants | ||||||
| Fel d1 in dust (>1 μg/g of dust) | 358 | 12 | 17.1 | 62 | 21.5 | ns |
| Fel d1 in dust (>8 μg/g of dust) | 358 | 5 | 7.1 | 20 | 6.9 | ns |
| Der p1 in dust (>2 μg/g of dust) | 359 | 17 | 24.3 | 83 | 28.7 | ns |
| Der p1 in dust (>10 μg/g of dust) | 359 | 4 | 5.7 | 28 | 9.7 | ns |
| NO2 levels (>45 ppb) | 347 | 39 | 59.1 | 146 | 52.0 | ns |
| Pets at home in 1st year (yes) | 391 | 23 | 30.7 | 117 | 37.0 | ns |
| Cord serum cotinine (>14 ng/ml) | 349 | 23 | 30.7 | 98 | 35.8 | ns |
| Atopy in the child | ||||||
| Total IgE in cord serum (>0.35 IU/ml) | 399 | 31 | 35.2 | 98 | 31.5 | ns |
LRTI indicates lower respiratory tract infection; ns, not significant; SGA, small for gestational age; socioeconomic level I, managers and occupations with higher university qualification; II, occupations with lower university qualification and senior technicians; III, occupations of administrative and financial management, and self-employed workers; IV, qualified and semi-qualified manual workers; V, unqualified workers; RSV, respiratory syncytial virus.
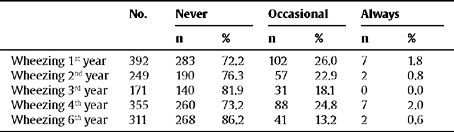
The prevalence of asthma at 6 years of age was 9.3%. Of these, for 26 children for whom complete data about asthma was available, it stands out that 5 (19.2%) were diagnosed with asthma in the first year of life, while the rest were diagnosed during the first 6 years of life. Table 2 details the frequency of wheezing in the last 12 months (never, occasional, always), depending on the year in which the interview was carried out. At the end of the study information was available about the presence of wheezing in each year of the follow-up for 368 children and this made it possible for them to be classified into different wheezing phenotypes: 191 children (51.9%) never suffered wheezing, 66 (17.9%) suffered early wheezing, 50 (13.6%) had late-onset wheezing, and 61 (16.6%) had persistent wheezing.
Frequency of suffering wheezing in the last 12 months, depending on the year of the interview
| No. | Never | Occasional | Always | ||||
| n | % | n | % | n | % | ||
| Wheezing 1st year | 392 | 283 | 72.2 | 102 | 26.0 | 7 | 1.8 |
| Wheezing 2nd year | 249 | 190 | 76.3 | 57 | 22.9 | 2 | 0.8 |
| Wheezing 3rd year | 171 | 140 | 81.9 | 31 | 18.1 | 0 | 0.0 |
| Wheezing 4th year | 355 | 260 | 73.2 | 88 | 24.8 | 7 | 2.0 |
| Wheezing 6th year | 311 | 268 | 86.2 | 41 | 13.2 | 2 | 0.6 |
A total of 149 children (30.6%) had a LRTI in the first year of life. The number of registered sets of clinical symptoms was 207, as the same child could have more than 1 LRTI during the monitoring period. The distribution of LRTIs was as follows: non-specific LRTI (11.0%), bronchitis (85.5%), laryngotracheobronchitis (5.0%), bronchiolitis (13.5%) and pneumonia (10.0%). It was possible to take nasopharyngeal samples from 99 children. 50% of the LRTIs with a mucus sample available gave positive results to some of the respiratory viruses studied, SRV being the most common (41%).
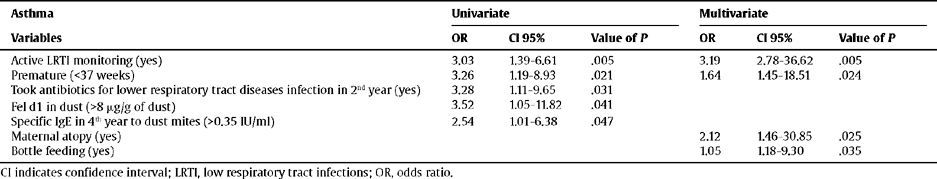
Relationship Between LRTIs and AsthmaThe univariate analysis highlights the following as risk factors for developing asthma at 6 years of age: having suffered a LRTI in the first year of life, prematurity, having taken antibiotics for a LRTI during the 2nd year of life, presence of cat epithelium allergens, and having high levels of specific IgE to mites in the 4th year of life. However, the final adjusted model only confirmed the following as risk factors; LRTIs, prematurity, being the child of a mother with atopy, and having been bottle fed (Table 3).
Relationship between the risk factors and asthma
| Asthma | Univariate | Multivariate | ||||
| Variables | OR | CI 95% | Value of P | OR | CI 95% | Value of P |
| Active LRTI monitoring (yes) | 3.03 | 1.39–6.61 | .005 | 3.19 | 2.78–36.62 | .005 |
| Premature (<37 weeks) | 3.26 | 1.19–8.93 | .021 | 1.64 | 1.45–18.51 | .024 |
| Took antibiotics for lower respiratory tract diseases infection in 2nd year (yes) | 3.28 | 1.11–9.65 | .031 | |||
| Fel d1 in dust (>8 μg/g of dust) | 3.52 | 1.05–11.82 | .041 | |||
| Specific IgE in 4th year to dust mites (>0.35 IU/ml) | 2.54 | 1.01–6.38 | .047 | |||
| Maternal atopy (yes) | 2.12 | 1.46–30.85 | .025 | |||
| Bottle feeding (yes) | 1.05 | 1.18–9.30 | .035 | |||
CI indicates confidence interval; LRTI, low respiratory tract infections; OR, odds ratio.
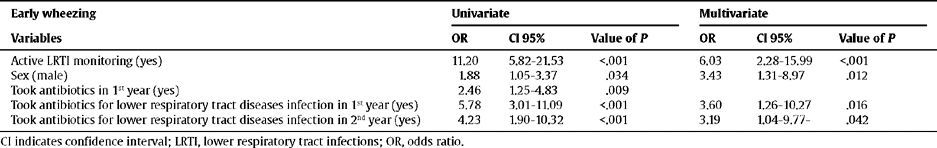
The children with a higher risk of having early wheezing were those who had LRTIs, were male, and had taken antibiotics for lower respiratory tract illnesses in the first 2 years of life (Table 4).
Relationship between the risk factors and wheezing
| Early wheezing | Univariate | Multivariate | ||||
| Variables | OR | CI 95% | Value of P | OR | CI 95% | Value of P |
| Active LRTI monitoring (yes) | 11.20 | 5.82–21.53 | <.001 | 6.03 | 2.28–15.99 | <.001 |
| Sex (male) | 1.88 | 1.05–3.37 | .034 | 3.43 | 1.31–8.97 | .012 |
| Took antibiotics in 1st year (yes) | 2.46 | 1.25–4.83 | .009 | |||
| Took antibiotics for lower respiratory tract diseases infection in 1st year (yes) | 5.78 | 3.01–11.09 | <.001 | 3.60 | 1.26–10.27 | .016 |
| Took antibiotics for lower respiratory tract diseases infection in 2nd year (yes) | 4.23 | 1.90–10.32 | <.001 | 3.19 | 1.04–9.77- | .042 |
CI indicates confidence interval; LRTI, lower respiratory tract infections; OR, odds ratio.
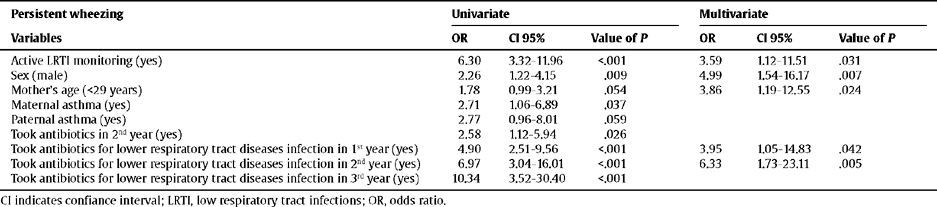
The children with persistent wheezing had LRTI, were male, were the children of young mothers (under 29 years), and had taken antibiotics for lower respiratory tract illnesses (Table 5).
Relationship between the risk factors and persistent wheezing
| Persistent wheezing | Univariate | Multivariate | ||||
| Variables | OR | CI 95% | Value of P | OR | CI 95% | Value of P |
| Active LRTI monitoring (yes) | 6.30 | 3.32–11.96 | <.001 | 3.59 | 1.12–11.51 | .031 |
| Sex (male) | 2.26 | 1.22–4.15 | .009 | 4.99 | 1.54–16.17 | .007 |
| Mother's age (<29 years) | 1.78 | 0.99–3.21 | .054 | 3.86 | 1.19–12.55 | .024 |
| Maternal asthma (yes) | 2.71 | 1.06–6.89 | .037 | |||
| Paternal asthma (yes) | 2.77 | 0.96–8.01 | .059 | |||
| Took antibiotics in 2nd year (yes) | 2.58 | 1.12–5.94 | .026 | |||
| Took antibiotics for lower respiratory tract diseases infection in 1st year (yes) | 4.90 | 2.51–9.56 | <.001 | 3.95 | 1.05–14.83 | .042 |
| Took antibiotics for lower respiratory tract diseases infection in 2nd year (yes) | 6.97 | 3.04–16.01 | <.001 | 6.33 | 1.73–23.11 | .005 |
| Took antibiotics for lower respiratory tract diseases infection in 3rd year (yes) | 10.34 | 3.52–30.40 | <.001 | |||
CI indicates confiance interval; LRTI, low respiratory tract infections; OR, odds ratio.
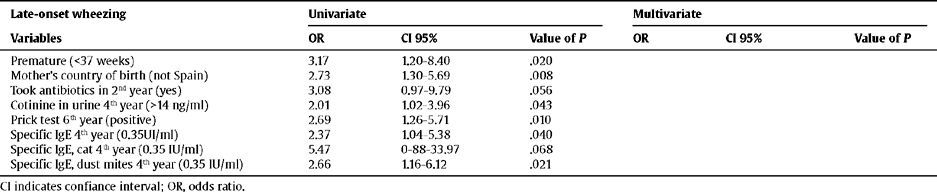
However, no relationship was found between LRTIs and late-onset phenotype wheezing (Table 6). The data suggest that the children most likely to develop late-onset wheezing: were premature, had an immigrant mother, were exposed to environmental tobacco smoke at 4 years of age, were sensitive to cat epithelium and dust mites, as well as having positive skin tests, and had taken antibiotics (not only for lower respiratory tract illnesses). However, none of these associations held up in the multivariate analysis.
Relationship between the risk factors and late-onset wheezing
| Late-onset wheezing | Univariate | Multivariate | ||||
| Variables | OR | CI 95% | Value of P | OR | CI 95% | Value of P |
| Premature (<37 weeks) | 3.17 | 1.20–8.40 | .020 | |||
| Mother's country of birth (not Spain) | 2.73 | 1.30–5.69 | .008 | |||
| Took antibiotics in 2nd year (yes) | 3.08 | 0.97–9.79 | .056 | |||
| Cotinine in urine 4th year (>14 ng/ml) | 2.01 | 1.02–3.96 | .043 | |||
| Prick test 6th year (positive) | 2.69 | 1.26–5.71 | .010 | |||
| Specific IgE 4th year (0.35UI/ml) | 2.37 | 1.04–5.38 | .040 | |||
| Specific IgE, cat 4th year (0.35 IU/ml) | 5.47 | 0-88–33.97 | .068 | |||
| Specific IgE, dust mites 4th year (0.35 IU/ml) | 2.66 | 1.16–6.12 | .021 |
CI indicates confiance interval; OR, odds ratio.
This study was carried out to identify the effect of LRTIs on asthma and wheezing, and to monitor the numerous covariables collected at different moments during the monitoring.
The prevalence of asthma obtained is comparable to that of other Mediterranean countries, but lower than in Anglo-Saxon countries. In Spain, the prevalence of asthma at 6 years of age ranges between 7% and 13%, according to the data of the ISAAC-III study in 2002.13 With regard to the prevalence of wheezing phenotypes in children under 6, our results are similar to those of Martinez et al2 in the Tucson cohort.
Relationship Between LRTIs and Asthma Diagnosis and Wheezing PhenotypeThere was a strong relationship between LRTIs and a diagnosis of asthma at 6 years of age, in accordance with the results of other authors.20–23 Furthermore, this study shows that there is also a relationship between having a LRTI during the first year of life and suffering from early and persistent wheezing. However, we found no relationship between RSV infection and asthma, as reported by some prospective studies.24,25 This is likely due to the small number of samples as active monitoring of respiratory symptoms was only carried out in the first year of life.
Several hypotheses have been proposed to explain the relationship between LRTIs and asthma. The first suggests that viral infections could affect the development of the immune response, or interfere with normal lung development and the regulation of the tone of the respiratory tracts. In this line, the viruses would be the causal agents in the onset of asthma (direct causation). A second hypothesis states that the viruses are triggered by the obstruction in the respiratory tract in subjects who already have prior functional or structural alteration of the airways or a susceptibility to developing an immune response which is predisposed to the obstruction (reverse causation). Different studies endorse this view because children with a family history of asthma and atopy have more LRTIs than those without.20 The third and most recent hypothesis, proposed by Martinez,26 states that the response to the different viruses associated with asthma depends on genetic history, exposure to other environmental factors, and the level of maturity of the respiratory and immunes systems. This would explain the observations in the asthma-virus relationship, that there is more than one long-term pattern for the response and consequences.
Relationship Between Covariables and Asthma Diagnosis at 6 Years of AgeBesides LRTIs, other variables found to be independent risk factors are: prematurity, bottle feeding and maternal atopy. Several authors point out that prematurity can cause reduced pulmonary development,27 lessening the calibre of the respiratory tract, which generates an increase in wheezing during respiratory infections and in turn increases asthma diagnoses. However, other authors have not found this association, arguing that it is difficult to separate the effects of prematurity per se from the effects associated with the mechanical ventilation that some of these children need during the perinatal period. In view of this discrepancy, a recent systematic review of the topic including a meta-analysis28 concludes that an association, although weak, does exist.
In our study, the other variable associated with the development of asthma is bottle feeding, which coincides with data from other authors.29 Bifidobacteria and lactobacillus, the predominant intestinal flora in children who were breast-fed, have been considered to be beneficial for obtaining a protective immune response to allergies. Recently, Frediksson et al30 found that breastfeeding and asthma has a U-shaped relationship. That is, the lowest prevalence of asthma is found in children who were breast-fed between 4 and 6 months of age, and the highest presence of chronic respiratory symptoms in children who were breast-fed between 7 and 9 months of age. Although epidemiological studies can provide contradictory information, the recommendation is still to breast-feed because of its other proven beneficial effects with regard to neurological development, obesity and hypertension.
With regard to the influence of a family history of asthma or atopy, conclusive evidence does not exist. Some studies show that maternal asthma is a risk,31 while others point out the opposite.32 In our study, there is a relationship between children diagnosed with asthma at 6 years of age and a maternal history of atopy. According to Ying et al,33 this relationship exists because the local response of the respiratory epithelium is affected in asthmatic children.
Relationship Between Covariables and WheezingSeveral epidemiological studies have confirmed that asthma and atopy are more common in males before puberty. This study can not confirm this relationship in children diagnosed with asthma, but can in those with early and persistent wheezing. After puberty it becomes more common among girls. Some authors have determined that this effect is due to testosterone being more anti-inflammatory than oestrogen.34 Other authors have found that the airway in boys is less developed than their lung volume, which is what has been called disynaptic lung growth.35
Recently, interest has been aroused in determining if a causal relationship exists between the use of antibiotics and the development of asthma or atopy. In our study we found a relationship in the children between taking antibiotics and having early and persistent wheezing. One explanation of this association could be put down to memory bias. The parents of the children with asthma or wheezing in our study, having undergone exhaustive monitoring, would be more predisposed to remembering the exposure to certain factors, including taking antibiotics. This association was also confirmed by other authors in a recent meta-analysis.36 It is difficult to explain the possible underlying biological mechanism. If this is a causal relationship, the possibility has been considered that antibiotics could increase the risk of developing asthma and atopy because they eliminate the bacterial infections that predispose the development of immunity to Th1 lymphocytes.37 Another possibility is that antibiotics could affect the intestinal flora, which also modulates the immune system.
In this study, the children with persistent wheezing often had mothers under 29 years of age, compared with those who never had wheezing. It has been well documented that in adolescent women some metabolic factors and socioeconomic level are associated with more negative effects during pregnancy. However, in our study the age of the mother is a risk factor that is independent of other perinatal and sociodemographic factors collected, so we can not rule out the possibility that maternal age was a probable confusing variable of another undetermined social indicator.
Strengths and Limitations of the StudyResults from this cohort study are considered valid as they provide information about risk factors at an early age, and the study providing data regarding the sequence of events in the natural history of the asthma and wheezing. It assesses the role of LRTIs in the first year of life by active, prospective recording of these illnesses, in order to avoid parent's memory bias. The follow-up rate in the study is relatively high given the difficulty with drop-outs in cohort studies. The distribution of the variables studied are similar in the drop-out group and the one that remained in the study, so it seems that the drop-outs have not led to a relevant information bias.
In this type of study the initial sample size is important as it could be a limitation of the study. In our study, some of the factors associated with asthma or with the different wheezing phenotypes have association estimates with wide confidence intervals, which reveal that increasing the sample size is not enough to be more accurate. It may not be possible to generalize our results as the study involved a Mediterranean population with a high immigrant component and with extensive exposure to tobacco smoke (up to 60% of the children in the cohort were exposed to tobacco smoke from a mother or other relative that smoked).
Finally, from a clinical and preventative point of view, it is important to extend our knowledge of the pathogenesis of LRTIs and the development of asthma and wheezing, with the ultimate aim of being able to avoid, or reduce as far as possible, their progression. On this subject, the study provides evidence into the role of LRTIs during the first year of life in the development of asthma and wheezing during childhood. But furthermore, our results suggest that this is a complex relationship which probably depends on other factors and mechanisms, so further research is necessary.
ConclusionsThe results obtained in this study confirm that LRTIs occurring during the first year of life are related to the diagnosis of asthma and to the clinical phenotypes of early and persistent wheezing. These results are in accordance with the concept that LRTIs occurring during a critical period of development, such as the first years of life, play an important role in the later onset of asthma and wheezing.