The coexistence of potentially aggravating processes is common in asthmatics, particularly in patients with difficult control. The primary aim of this study is to ascertain whether comorbidity is more common in uncontrolled patients. As a secondary aim, we propose to evaluate the correlation between the asthma control test (ACT) and the fraction of exhaled nitric oxide (FENO).
Patient and methodsA prospective, observational study comparing the function and clinical picture of two groups of asthmatics: controlled (ACT≥20) and uncontrolled (ACT<20). They were all assessed for, smoking, rhinosinusitis, obesity, anxiety, depression, vocal chord dysfunction, gastro-oesophageal reflux (GORD), allergic bronchopulmonary aspergillosis (ABPA), COPD and nasal polyps.
ResultsA total of 50 patients with controlled asthma and 102 with sub-optimal control were included. The patients with an ACT≥20 had better lung function, less variation in PEF, less bronchial hyper-reactivity and lower FENO values. Comorbidities were found in 95% of the controlled asthmatics and in 97% of the uncontrolled. Only the presence of nasal polyps, GORD and ABPA was more frequent in the uncontrolled group. However, the simultaneous presence of 3 or more comorbidity factors was significantly more frequent in patients with sub-optimal control (P=.01). There was no significant correlation between the FENO and the ACT values (rho=−0.08; P=.32).
ConclusionsAggravating comorbidities are more common in patients with sub-optimal control. There was no correlation between the FENO and the ACT values.
La coexistencia de procesos potencialmente agravantes es frecuente en asmáticos, especialmente en individuos con control difícil. Es objetivo primario conocer si la comorbilidad es más frecuente en los pacientes no controlados. Como objetivo secundario nos propusimos evaluar el grado de correlación entre test de control del asma (ACT) y la fracción exhalada de óxido nítrico (FENO).
Pacientes y métodosEstudio prospectivo, observacional, que comparó funcional y clínicamente dos grupos de asmáticos: controlados (ACT ≥ 20) y no controlados (ACT < 20). En todos se investigó la presencia de tabaquismo, rinosinusitis, obesidad, ansiedad, depresión, disfunción de cuerdas vocales, reflujo gastroesofágico (RGE), aspergilosis broncopulmonar alérgica (ABPA), EPOC y poliposis nasal.
ResultadosSe incluyeron 56 pacientes con asma controlada y 102 con un control subóptimo. Lós pacientes con un ACT ≥ 20 tenían mejor función pulmonar, menor variabilidad del PEF, menos hiperreactividad bronquial y menores valores de FENO. Se hallaron comorbilidades en el 95% de los asmáticos controlados y en el 97% de los no controlados. Sólo la presencia de poliposis nasal, RGE y ABPA fue más frecuente en el grupo no controlado. Sin embargo, la presencia simultánea de 3 o más factores de comorbilidad fue significativamente más frecuente en los pacientes con un control subóptimo (P = 0,01). No hubo correlación significativa entre los valores del FENO y los del ACT (rho = −0,08; P = 0,32).
ConclusionesLa suma de comorbilidades agravantes es más frecuente en pacientes con control subóptimo. No existe correlación entre los valores de ACT y de FENO.
The primary goal of asthma treatment, according to the Spanish guidelines for asthma management (GEMA 2009), is to achieve and maintain disease control,1 which implies normal health-related quality of life.2 However, in a high number of patients, this objective is not usually achieved. A study by González-Barcala et al3 conducted in the field of primary care on a sample of 2159 asthmatics showed that 64% were poorly controlled.
There are many factors that explain poor control of asthma: sub-optimal doses of medication,4 poor compliance or inadequate inhalatory tecnique5 and an asthmatic phenotype that do not usually respond to medication6 are examples. In two series of patients with difficult to control asthma, concomitant processes were found that could aggravate the disease in 19–34% of cases.7,8 It is possible that the presence of comorbidity is associated with a poorer therapeutic response to the escalated treatment recommended for patients with poorly controlled asthma, but it is clear that the specific treatment of concurrent processes improves asthma control.8
In recent years, using a standardised methodology, instruments have been developed that are designed to measure asthma control from the perspective of the patient. Among them, the Asthma Control Test (ACT),9 whose Spanish-language version has been recently validated,10 has emerged as a simple and useful questionnaire for assessing the clinical status of asthmatics. Meanwhile, asthma specialists have been interested in finding an easily measurable and reproducible objective to facilitate monitoring of the disease and to avoid over or under treatment. Exhaled nitric oxide (FENO), a surrogate indicator of eosinophilic bronchial inflammation, has been used to guide asthma therapy.11,12 The relationship between the two types of instruments, clinical and biochemical, has rarely been studied and it has been suggested that it is possible that they measure different aspects of the disease.13
This study is based on the hypothesis that asthma control may be adversely affected by concurrent processes. Our primary objective was to know whether poorly controlled asthma is related to the presence of comorbidities. As a secondary objective, we studied whether the degree of control, measured by the ACT, correlates with FENO levels.
Material and MethodsStudy DesignProspective, observational study conducted in the respiratory and allergy departments of two hospitals. Patients who were regularly seen by asthma treatment specialists were recruited.
PatientsPatients were consecutively included who were diagnosed with poorly controlled asthma (defined by a value of <20 on the ACT) up to a count of 100. A control group was established including the first 50 patients with well-controlled asthma (ACT≥20 and no exacerbations in the last 3 months). The diagnosis of asthma was established by a pulmonologist or allergist specialist with experience with this disease. Patients were considered to be asthmatic if they had suggestive symptoms (intermittent coughing episodes, dyspnoea and wheezing) in addition to any of the following criteria: positive bronchodilator test, variability in peak expiratory flow≥20%, positive skin tests, positive methacholine test or high FENO. All patients included received treatment with at least one inhaled corticosteroid, and all those in the “uncontrolled” group used a combination of inhaled corticosteroid and long-term β2-agonist. All subjects included were educated about their disease and the correct use of inhaled medication during a nursing visit. The inclusion period lasted from 1 February until 24 June 2009. The study was submitted for approval by the Ethics Committees of each hospital and informed consent was provided by all participants.
ProtocolAll subjects filled out a detailed clinical history that specified tobacco habits, the coexistence of atopic traits, disease evolution, treatments, etc. Spirometry was performed with a bronchodilator test, a methacholine challenge test (if not contraindicated), a measurement of FENO, completion of the Goldberg questionnaire for anxiety and depression, and patients were instructed to perform an outpatient peak flow recording (PEF) for a month. A structured history was obtained with the aim of ruling out coexisting conditions that could worsen asthma control (obesity, tobacco smoking, smoke exposure, anxiety, depression, medications that could affect asthma control, gastro-oesophageal reflux, upper airway disease, vocal chord dysfunction, chronic obstructive pulmonary disease and allergic bronchopulmonary aspergillosis). It was noted which of these processes had been diagnosed and, in cases where there was an unconfirmed clinical suspicion, relevant tests were requested to confirm the diagnosis (eg. breast or chest CT, digestive endoscopy, etc.).
Measurement
Exhaled nitric oxideA single measurement was performed using a portable device (NIOX MINO; Aerocrine AB Solna: Sweden) at an oral flow of 50 ml/s for 10 seconds. The sensor device was changed periodically according to the manufacturer's instructions.
Pulmonary FunctionSpirometry was performed using a Datospir 120 spirometer (SIBELMED, Spain) according to the guidelines of the American Society/European Respiratory Society14 to calculate the forced expiratory volume in one second (FEV1), forced vital capacity (FVC), the FEV1/FVC ratio and peak expiratory flow (PEF). FEV1 and FVC values were expressed as a percentage of the theoretical (% pred). The bronchodilator test was calculated as the percentage change in FEV1 from baseline, 15 min after inhaling 400 μg salbutamol.
Bronchial hyperreactivity to methacholine (expressed as PC20methacholine) was measured using the tidal breathing method for two minutes, adapted from Cockcroft et al.15 Values of PC20≤8 mg/ml were interpreted as positive.
The PEF, measured every 12h with a portable device, was recorded by the patient in a log.
Asthma Control QuestionnaireWe evaluated asthma control by using the Spanish-language version of the validated Asthma Control Test (ACT).10 Patients subjectively assessed the degree of discomfort caused by asthma during the preceding four weeks by responding to five questions on a scale of five possible answers. The ACT is simple, it has been validated, and it varies with the changes in asthma control over time. A cut-off point of 19 or less identifies patients that have sub-optimal control.10
Goldberg Test of Depression and AnxietyThis test was designed to be used by non-psychiatric physicians in clinical research. The result reflects responses (‘yes’ or ‘no’) to 9 questions about anxiety and 9 about depression. It is only necessary to answer the complete block of 9 questions if one of the first 4 is answered in the affirmative, focusing on how the subject has felt during the preceding month. Goldberg et al considered that a result of ≥5 in the section on anxiety or ≥2 in the depression section implies a 50% chance of having a clinically relevant disorder.16
Statistical AnalysisData are presented as averages (standard deviation) for quantitative variables and as percentages for qualitative variables. To measure normality the Saphiro-Wilks test was used. Differences between quantitative variables were calculated with the Student's t test for independent data that follow a normal distribution, and the Wicoxon test for non-normally distributed data. Differences between percentages were calculated with Fisher's exact test. In the case of ordinal variables, the chi-squared test was used for tendencies. The presence of comorbidity factors in the two groups (controlled and uncontrolled patients) was compared using the odds ratio calculated from 2×2 tables, using controlled patients without comorbidity as a reference group. We used the Spearman rh0 rank correlation coefficient to measure the relation between ACT and FENO values. Results were considered statistically significant when P<.05. 95% confidence intervals are provided.
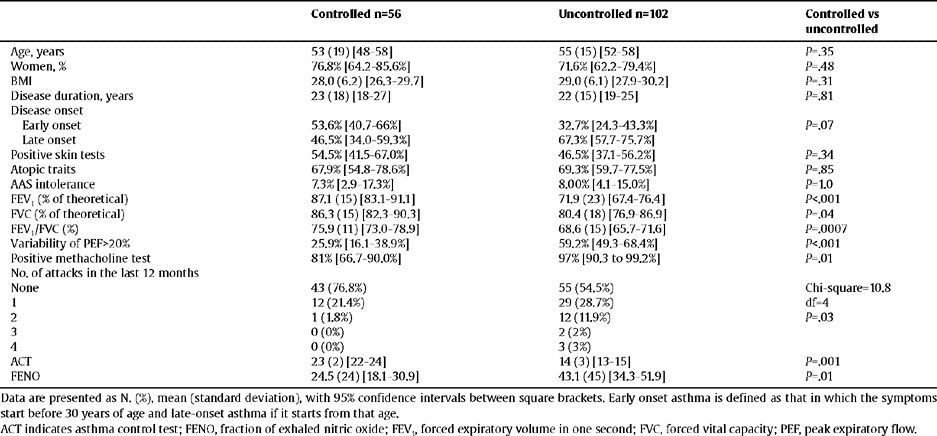
ResultsDemographic CharacteristicsWe included 158 patients, divided into 2 groups: 56 with controlled asthma and 102 with sub-optimal control. The demographic, clinical and functional characteristics are shown in table 1, together with the comparison between the two groups of patients. No significant differences were found in age, gender, nor BMI. Patients with an ACT≥20 had better lung function, lower PEF variability, less bronchial hyperreactivity and lower values of FENO.
Demographic characteristics. Clinical and functional characteristics of patients included in the study according to the degree of asthma control
| Controlled n=56 | Uncontrolled n=102 | Controlled vs uncontrolled | |
| Age, years | 53 (19) [48–58] | 55 (15) [52–58] | P=.35 |
| Women, % | 76.8% [64.2–85.6%] | 71.6% [62.2–79.4%] | P=.48 |
| BMI | 28.0 (6.2) [26.3–29.7] | 29.0 (6.1) [27.9–30.2] | P=.31 |
| Disease duration, years | 23 (18) [18–27] | 22 (15) [19–25] | P=.81 |
| Disease onset | |||
| Early onset | 53.6% [40.7–66%] | 32.7% [24.3–43.3%] | P=.07 |
| Late onset | 46.5% [34.0–59.3%] | 67.3% [57.7–75.7%] | |
| Positive skin tests | 54.5% [41.5–67.0%] | 46.5% [37.1–56.2%] | P=.34 |
| Atopic traits | 67.9% [54.8–78.6%] | 69.3% [59.7–77.5%] | P=.85 |
| AAS intolerance | 7.3% [2.9–17.3%] | 8.00% [4.1–15.0%] | P = 1.0 |
| FEV1 (% of theoretical) | 87.1 (15) [83.1–91.1] | 71.9 (23) [67.4–76.4] | P<.001 |
| FVC (% of theoretical) | 86.3 (15) [82.3–90.3] | 80.4 (18) [76.9–86.9] | P=.04 |
| FEV1/FVC (%) | 75.9 (11) [73.0–78.9] | 68.6 (15) [65.7–71.6] | P=.0007 |
| Variability of PEF>20% | 25.9% [16.1–38.9%] | 59.2% [49.3–68.4%] | P<.001 |
| Positive methacholine test | 81% [66.7–90.0%] | 97% [90.3 to 99.2%] | P=.01 |
| No. of attacks in the last 12 months | |||
| None | 43 (76.8%) | 55 (54.5%) | Chi-square=10.8 |
| 1 | 12 (21.4%) | 29 (28.7%) | df=4 |
| 2 | 1 (1.8%) | 12 (11.9%) | P=.03 |
| 3 | 0 (0%) | 2 (2%) | |
| 4 | 0 (0%) | 3 (3%) | |
| ACT | 23 (2) [22–24] | 14 (3) [13–15] | P=.001 |
| FENO | 24.5 (24) [18.1–30.9] | 43.1 (45) [34.3–51.9] | P=.01 |
Data are presented as N. (%), mean (standard deviation), with 95% confidence intervals between square brackets. Early onset asthma is defined as that in which the symptoms start before 30 years of age and late-onset asthma if it starts from that age.
ACT indicates asthma control test; FENO, fraction of exhaled nitric oxide; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; PEF, peak expiratory flow.
Data are presented as No. (%), mean (SD), with 95% confidence intervals in brackets. Early onset asthma is defined as symptoms beginning before 30 years of age and late onset if symptoms begin after this age.
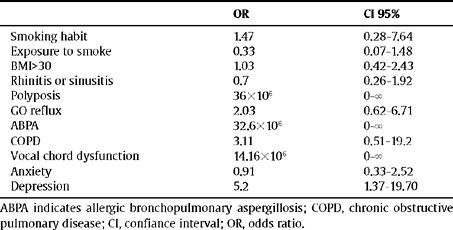
Presence of ComorbidityThe presence of some comorbidity factor (smoking habit, exposure to smoke, obesity, rhinitis or sinusitis, nasal polyposis, gastro-oesophageal reflux, allergic bronchopulmonary aspergillosis, COPD, vocal chord dysfunction, anxiety or depression) was found in 95% [95% CI 85.4 to 98.2%] of controlled asthmatics and in 97% [95% CI 91.7 to 99] of those who had not achieved control. There were no significant differences in BMI respectively comparing their various ranges in controlled and uncontrolled patients, BMI<30: 64.3% [95% CI 50.4 to 76.6] vs 57.8% [95% CI 48.2 to 67.4]; BMI between 30 and 35: 21.4% [95% CI 12.0 to 35.6] vs 22.5% [95% 14.4 to 30 7], BMI>35: 14.3% [95% CI 6.6 to 27.1] vs 19.6% [95% CI 11.9 to 27.3]. Only the presence of nasal polyposis: 0% [95% CI 0 to 5.5] vs 8.8% [95% CI 4.7 to 15.9] (P=.03), the coexistence of gastro-oesophageal reflux: 8.9% [95% CI 3.9 to 19.3] vs 22.8% [95% CI 15.5 to 31.6] (P=0.3), and allergic bronchopulmonary aspergillosis: 0% [95% CI 0 to 6.0] versus 9.8% [95% CI 5.4 to 17.1] (P=.03) were more frequent in the uncontrolled group.
Table 2 presents, expressed as OR, the prevalence of various comorbidities between the two groups.
Frequency of diagnosis of the various comorbidity factors in the uncontrolled group of patients with respect to the controlled group
| OR | CI 95% | |
| Smoking habit | 1.47 | 0.28–7.64 |
| Exposure to smoke | 0.33 | 0.07–1.48 |
| BMI>30 | 1.03 | 0.42–2.43 |
| Rhinitis or sinusitis | 0.7 | 0.26–1.92 |
| Polyposis | 36×106 | 0–8 |
| GO reflux | 2.03 | 0.62–6.71 |
| ABPA | 32.6×106 | 0–8 |
| COPD | 3.11 | 0.51–19.2 |
| Vocal chord dysfunction | 14.16×106 | 0–8 |
| Anxiety | 0.91 | 0.33–2.52 |
| Depression | 5.2 | 1.37–19.70 |
ABPA indicates allergic bronchopulmonary aspergillosis; COPD, chronic obstructive pulmonary disease; CI, confiance interval; OR, odds ratio.
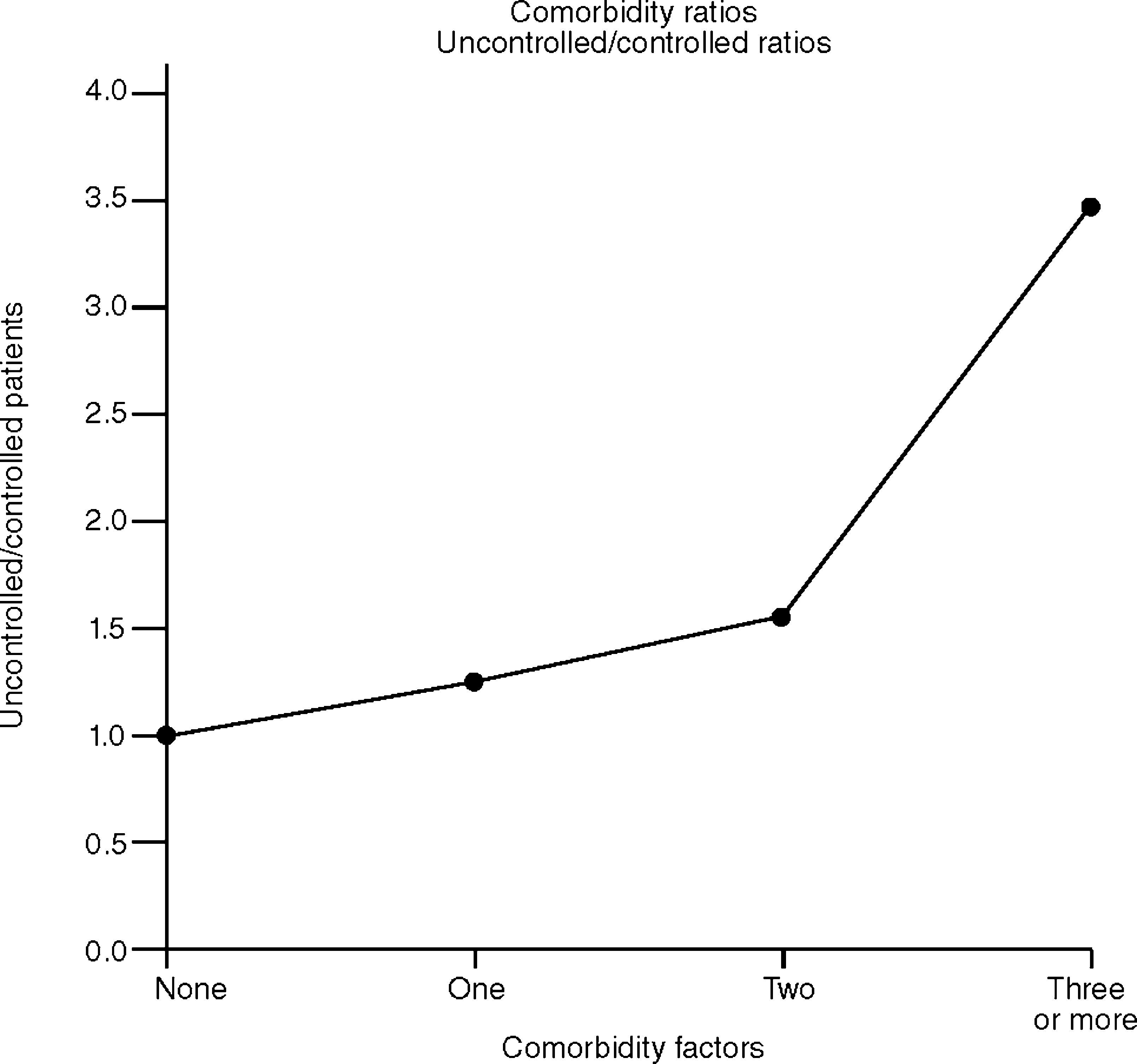
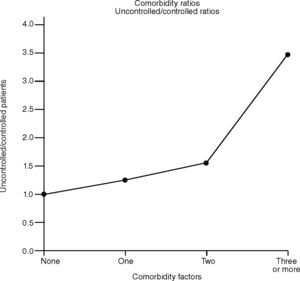
However, the simultaneous presence of 3 or more comorbidity factors was significantly more frequent in patients with sub-optimal control (P=.01). The ratios of patients with sub-optimal control with respect to those with control were: 1.24 in the case of a single comorbidity process, 1.53 in the case of two and 3.46 in the case of three or more comorbidities. This shows the tendency of uncontrolled patients to have more aggravating factors than those who have gained control of asthma (fig. 1).
Correlation between FENO and ACTAs expected, the FENO values were significantly lower in controlled patients: 24.5 [CI 95% 18.1 a 30.9] vs 43.1 [CI 95% 34.3–51.9]; P=.01. However, no significant correlation was found between the FENO values and ACT values when all patients included in the study were evaluated (rho=−0.08; P=.32).
DiscussionThe results of this study show the high percentage (>90%) of asthmatic patients who have comorbidities likely to worsen asthma control. Consequently, there are no significant differences in incidence between controlled asthmatic patients and those who are not (95% vs 97%) if comorbidity is defined as the presence of at least one potentially aggravating factor. However, the study shows that the coexistence in the same patient of several comorbidities is more common in cases of poorly controlled asthma (23.2% of controlled patients showed three or more comorbidities, while the same occurs in 44% of uncontrolled patients.)
There is increasing interest in the influence of comorbidity on the development and control of asthma17 and it has been suggested that the identification of these factors be part of the routine evaluation of asthma patients, particularly those that have difficulty controlling their asthma. An association between asthma severity and response to treatment and the following conditions has been reported: gastro-oesophageal reflux,18,19 obesity,20,21 vocal chord dysfunction,22 anxiety-depression,23,24 rhinosinusitis25,26 and smoking habit.27 However, the influence of these diseases on the course of asthma is variable and, in many cases, is not clear at all. Theoretically, it could alter the asthma phenotype (e.g. tobacco smoking, ABPA), be part of the same pathophysiological process (e.g. nasosinusitis) or could act as confounding factors in the diagnosis or assessment of asthma control (e.g. anxiety and depression). However, the impact of any of these processes in each individual patient is different and therefore difficult to predict. Our results show that the vast majority of asthmatics have some concomitant disease that can affect asthma evolution, whatever may be the degree of control. Only nasal polyposis (8.8 vs 0%), gastro-oesophageal reflux (22.8 vs 8.9%) and ABPA (9.8 vs 0%) were more frequent in uncontrolled patients (depression did not reach statistical significance by a small margin.) Patients with poor control show a clear trend towards having more comorbidities at the same time.
From these results we can get two obvious practical consequences. On the one hand, if the presence of comorbidities is associated with poor asthma control, the specific treatment of them could result in a benefit in patient quality of life. However, this is not always the case, at least not in the case of subclinical gastro-oesophageal reflux diagnosed by pH monitoring. The study of The American Lung Association Asthma Clinical Research Centers showed that treatment with esomeprazole for asthmatics with this concomitant process did not improve control of the disease.28 On the other hand, the co-existence of processes that potentially exacerbate asthma, but that have a different biological nature, can make an increase in medication based on the recommendations of the GEMA ineffective because the patient's symptoms would not be directly related to the bronchial inflammatory process. If this is the situation, we find cases of excessive and inappropriate treatment. At this point it would be very useful to have a marker to predict response to treatment, distinguishing between under-treated patients and those for whom increasing specific therapeutic strategies for asthma would be superfluous. Our group has shown that elevated FENO values imply that asthma control can be achieved with an increase in medication. In contrast, a poorly controlled patient with normal values of FENO is unlikely to improve with further treatment for asthma,29 which would result in a decrease in therapeutic expectations, at least if we base the concept of asthma control on symptom questionnaires.
The ACT has proved to be a reliable instrument to measure asthma control.9 However, it is based on the subjective perception that a patient may have regarding their symptoms and the answer to any of the questions included. For example, “during the past four weeks, how often have you noticed that you are short of breath?” can be influenced by concurrent processes that can cause similar symptoms (e.g., obesity, anxiety, etc.) Thus, it is not surprising that we found no correlation between ACT values and the FENO values. The same conclusion was reached by Shirai et al in a study with fewer patients and which did not emphasise the presence of comorbidity.13 Since they found high levels of FENO in patients with asthma control, they speculated that the presence of underlying eosinophilic inflammation (what FENO would indirectly show) is not an obstacle for the medication to eliminate symptoms. In our series it was observed that the FENO value was significantly higher in the group of patients with sub-optimal control (43.1 vs 24.5ppb), and the finding of cases with high FENO and an ACT≥20 was very rare. Although the hypotheses of Shirai were true, we believe it is too simple to explain the discrepancy between FENO and ACT. Patients with difficult to control asthma are a heterogeneous group and it is possible that our series included neutrophilic phenotype individuals or patients whose symptoms corresponded to the presence of comorbidity, both situations in which the ACT could be low and the FENO normal.
The originality of this study is that we have arranged the groups according to the degree of asthma control, something that had not been done in previous studies which focused on the potential effect of comorbidity on asthma. This allowed us to bring our approach to the objectives of the latest version of the GEMA – targeting control as a desirable objective – and to speculate on the potential limitations of the ACT, a tool widely used in clinical practice. Establishing a causal link between the presence of comorbidity and poor control of asthma will not be easy, as there are potential misleading factors that make the results difficult to interpret.
A potential limitation of this study is the number of patients that, although higher than in similar studies, may not be sufficient to demonstrate a statistical relationship between asthma control and certain processes that, like depression, show a tendency to reach significance in our results. Another shortcoming lies in the fact that the diagnosis of gastro-oesophageal reflux was purely clinical and pH monitoring or endoscopy was performed in all cases, something that presumably would have allowed the identification of sub-clinical cases.
The findings of this study are: the vast majority of patients with asthma, be it well or poorly controlled, present a process that is likely to worsen the control of the disease; patients with sub-optimal control show a tendency to accumulate comorbidities; there is no correlation between ACT and FENO, meaning that these tools reflect different clinical realities.
Contributions
GSK provided the nitric oxide analysers used in this study. This funding source did not participate in the study design or in collecting the data, analysis or interpretation. Neither has been linked to the drafting of the manuscript or the decision to publish.
Conflict of Interest
Dr. Luis Pérez de Llano has received financial compensation from the GSK laboratories and Astra for participating in courses and giving talks on asthma.