Non-pharmacological treatment is essential in patients with chronic obstructive pulmonary disease (COPD), but this treatment is sometimes not given the importance it deserves. Patients diagnosed with COPD should benefit from comprehensive care services. These services comprise a protocolized set of actions aimed at covering the health needs of the patient, taking into account their environment and circumstances. Pulmonary rehabilitation is one of the essential components of non-pharmacological treatment in comprehensive COPD care services. In the Spanish COPD Guidelines (GesEPOC) 2017, we provided a systematic report of the scientific evidence for pulmonary rehabilitation programs in acute and stable phase disease. Another important issue in the non-pharmacological treatment of COPD is physical activity, and the most essential considerations regarding prescription are described in the GesEPOC guidelines, along with a review of the most effective strategies to ensure adherence. GesEPOC 2017 aims to underline the importance of non-pharmacological treatment as a co-adjuvant to pharmacological treatment.
El tratamiento no farmacológico es fundamental en los pacientes con enfermedad pulmonar obstructiva crónica (EPOC), sin embargo, este tratamiento, en ocasiones, no recibe la importancia que merece. Los pacientes diagnosticados de EPOC deberían beneficiarse de servicios de atención integral. Estos servicios son un conjunto articulado de acciones estandarizadas dirigidas a la cobertura de las necesidades de salud del paciente, considerando el entorno y las circunstancias. La rehabilitación pulmonar es uno de los componentes esenciales del tratamiento no farmacológico en los servicios de atención integral en la EPOC. En la Guía española de la EPOC (GesEPOC) 2017 detallamos de forma sistemática la evidencia científica de los programas de rehabilitación pulmonar en fase aguda y estable. Otro aspecto importante del tratamiento no farmacológico es la actividad física y en la guía GesEPOC 2017 describimos los puntos más esenciales sobre su prescripción y revisamos las estrategias más eficaces para su adhesión. GesEPOC 2017 quiere dejar constancia de la importancia del tratamiento no farmacológico como coadyuvante al tratamiento farmacológico.
The Spanish Chronic Obstructive Pulmonary Disease guidelines (GesEPOC) define chronic obstructive pulmonary disease (COPD) as a respiratory disease characterized by persistent symptoms and chronic airflow limitation, caused mainly by smoking.1
The emergence of new drugs and safer and more effective inhalation devices has sparked interest in the pharmacological treatment of COPD patients. This pharmacological treatment, however, must be associated with non-pharmacological treatment that is sometimes sidelined, or even completely ignored.
The recently updated GesEPOC guidelines1place emphasis on the importance of integrated care, including non-pharmacological treatment, in which pulmonary rehabilitation (PR) is considered an essential element of integrated care strategies (ICS).
The methodology used to produce the guidelines meets the quality standards for the development of clinical practice guidelines, and has been described in a recent publication.1 In this article, we will discuss the care model for patients with chronic diseases, specifically adapted to COPD. PR, physical activity (PA), self-care strategies, structured therapeutic education (TE) programs, and nutrition will all be addressed.
The Integrated Care Model in Chronic Obstructive Pulmonary DiseaseWhat are the Essential Elements?Current health systems are designed to treat and resolve acute episodes, but integral care of the patient is not an objective. These models are generally fragmented and multidisciplinary intervention is rare. They are oriented toward conventional hospital admission and follow-up in hospital outpatient units and primary care. Alternatives to conventional hospitalization, such as hospital-at-home and domiciliary care of complex patients are rarely considered, despite their proven cost-effectiveness. Clinical guidelines for diseases do not address systemic manifestations and common comorbidities that often contribute to the burden of disease. COPD patients have complex health needs, and the health system must be redesigned to meet these requirements.
Complexity is difficult to define, measure or predict. The term “patient complexity” applies to individuals who may fulfill at least one of the following criteria: (a) need for follow-up by different specialists; (b) frailty, requiring additional help due to functional decline and social deficits or temporary situations, such as hospital discharge, and (c) need for highly specialized care at home.
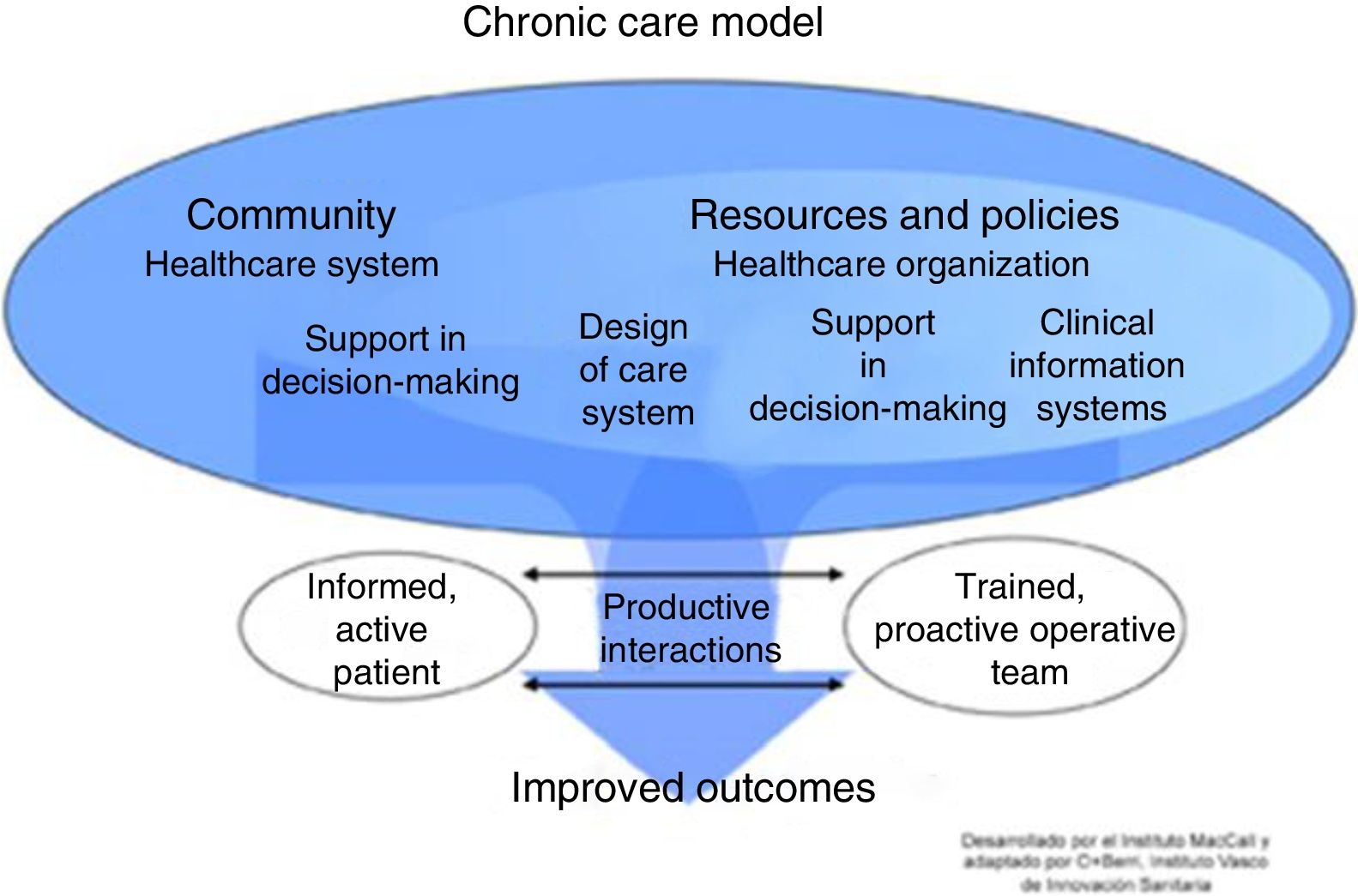
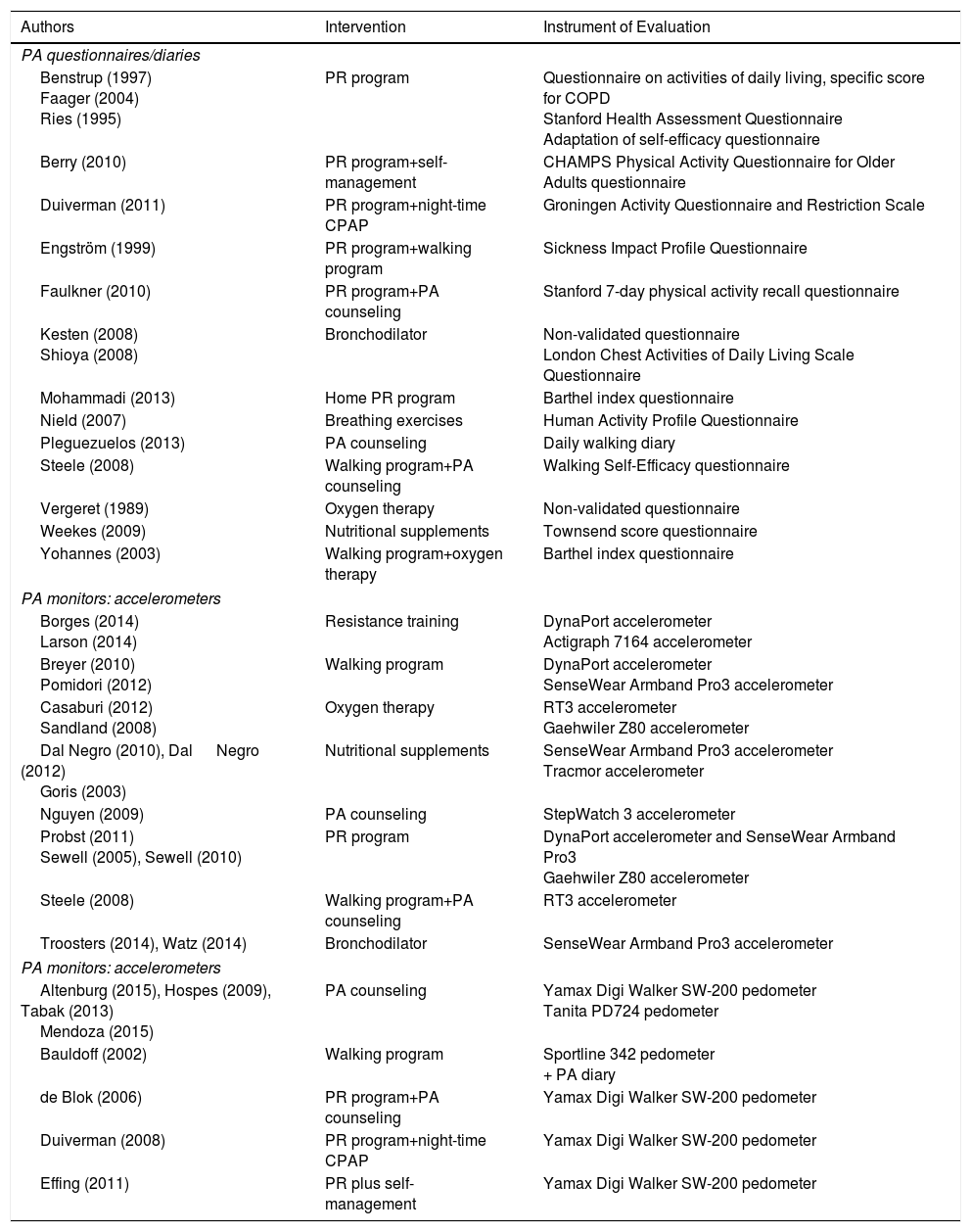
The progress of patients with complex needs often fluctuates, so the type of follow-up and the professionals involved may vary. Innovative services with technological support that improve efficiency and contribute to a better understanding of the underlying mechanisms of chronic diseases are required.2–4 There is no universal model for the management of chronic diseases, but conceptual frameworks exist that have demonstrated better outcomes.5 The most widely used is the Chronic Care Model (CCM) proposed by the World Health Organization (WHO) (Fig. 1). One of the main components of this model is ICS. Nevertheless, the effectiveness of CCM in COPD is limited and variable, and depends on the type of interventions and the experience of the professionals involved.6,7
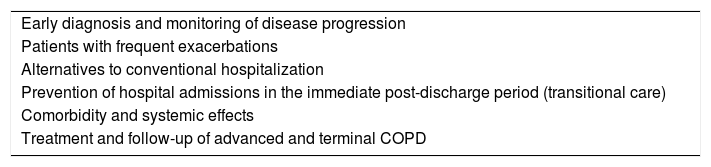
Integrated Care StrategiesAn ICS is a protocolized set of actions aimed at meeting the health needs of patients, taking into account their setting and circumstances. It proposes health objectives in the framework of a global patient plan, not necessarily restricted to a specific disease. It is designed around a continuum of processes provided by each service over a specific period of time. The results of implementing ICS in clinical practice have been inconclusive.8 Data from professionals, patients and biomedical research must be pooled in order to assess the risk in the prevention or reduction of the impact of the disease. Clinical challenges of COPD in ICS are specified in Table 1.
Clinical Challenges of COPD in an Integrated Care Strategy.
| Early diagnosis and monitoring of disease progression |
| Patients with frequent exacerbations |
| Alternatives to conventional hospitalization |
| Prevention of hospital admissions in the immediate post-discharge period (transitional care) |
| Comorbidity and systemic effects |
| Treatment and follow-up of advanced and terminal COPD |
According to the WHO,9 the patient must be evaluated according to 6 dimensions (sociodemographic; healthcare model and type of professionals involved; chronic disorders and risk factors; drug treatment; non-pharmacological interventions; and care facility) before any intervention can be proposed. ICS deployment strategies require site-specific adaptations and must be developed in sequential building blocks.10
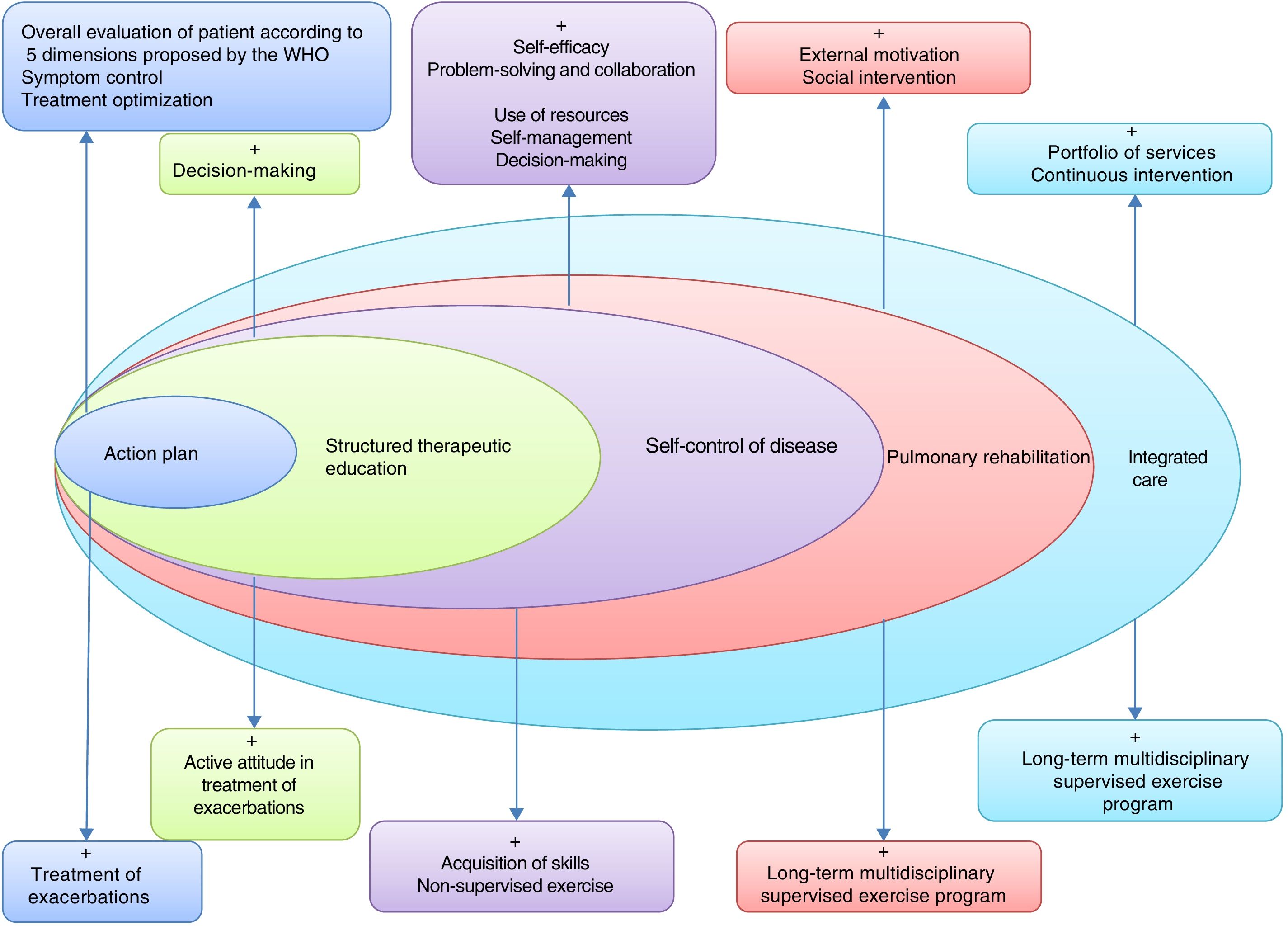
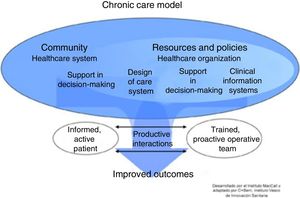
This will require the development of tools to assess individual risk, new training modalities for professionals, and instruments to evaluate the application of these strategies in the different territories. Fig. 2 lists the basic components of a proposed ICS in COPD.
Basic elements of an integrated care model in COPD. Adapted by C. Hernández de Spruit et al.11
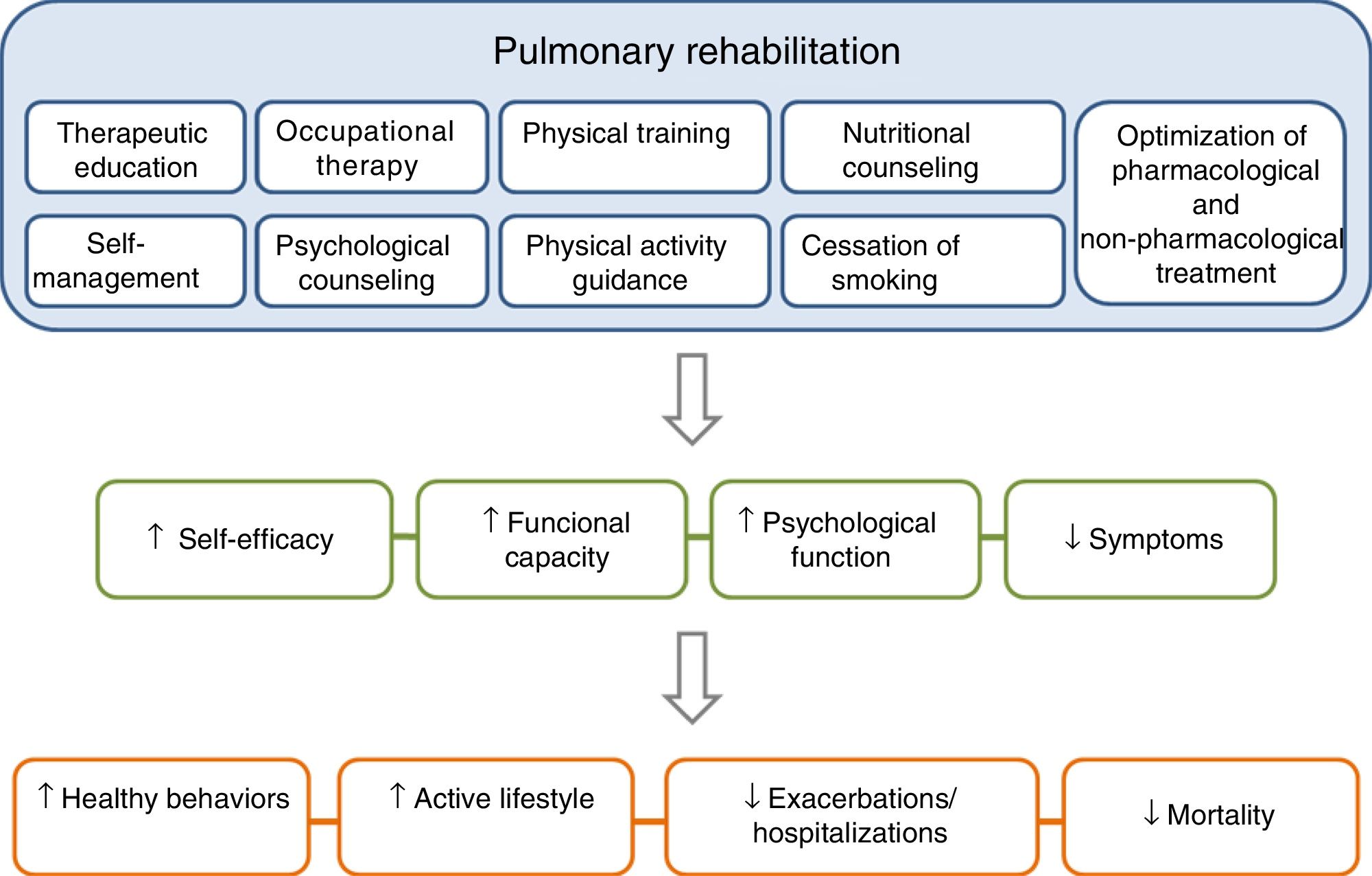
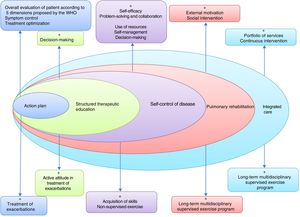
PR is one of the essential components of ICS in COPD, and its effects are well established11–15 (Fig. 3). The concept of PR from the American Thoracic Society (ATS) and the European Respiratory Society (ERS) consensus was incorporated into the 2014 update of the GesEPOC guidelines, placing more emphasis on its central role in the integrated care of the patient with chronic disease.11,16 The ATS and the ERS have also developed guidelines aimed at improving the implementation of PR.11,12,17 The new definition describes PR as a “comprehensive intervention based on a thorough patient assessment followed by patient-tailored therapies that include, but are not limited to, exercise training, education, and behavior change, designed to improve the physical and psychological condition of people with chronic respiratory disease and to promote the long-term adherence to health-enhancing behaviors”.11
The components of a complete, integrated pulmonary rehabilitation program have a direct and positive physical and psychological effect on patients, helping them to become more proactive toward their disease, acquiring healthy lifestyle behaviors, thus reducing the risk of exacerbations and mortality. Adapted from Spruit et al.27
The latest evidence and recommendations for ordering PR are summarized below:
- –
Results from different studies recommend that all COPD patients are included in a PR program as part of their treatment.12
- –
Structured TE programs should begin at the time of diagnosis and continue for the rest of the patient's life. Programs will be adapted to the needs of the patient and comorbidities will be taken into account.18
- –
Prescribing PR in patients with mild COPD is considered a weak recommendation, despite improvements in variables such as quality of life and exercise capacity.19
- –
Physical exercise, started during or immediately after a COPD exacerbation, improves quality of life and exercise capacity; however, the wide range of PR programs on offer may explain the heterogeneous effect on outcomes such as hospital readmissions and mortality.20 Doubts persist regarding the ideal timing of PR, and until more reliable information is available, the initiation of PR during hospital admission should be avoided.20
- –
Limited access to PR for COPD patients is prompting further research into community-based programs that require less infrastructure.11,21,22
- –
Intervention aimed at changing behaviors can help improve participation in COPD self-management programs.23,24
- –
Understanding of the effects of technology applied to physical training, TE, disease management, and PA in the PR setting is still limited.25–27
- –
Psychosocial factors and the perceptions of patients themselves can contribute to the adherence and maintenance of the effects of PR in the long term.23
- –
PR is safe, given the low incidence of major complications.28
PA must be considered a vital necessity for COPD patients. However, PA is far from simple, and is influenced by multiple factors that affect the patient's ability to adhere to the corresponding programs. PA must be prescribed as part of a change in lifestyle for COPD patients.
How Should Physical Activity be Prescribed and What Strategies can be Used to Encourage the Chronic Obstructive Pulmonary Disease Patient to do Physical Activity?The importance of prescribing PA in COPD patients is determined, among other factors, by: (a) the high morbidity and mortality associated with COPD29; (b) evidence that physical inactivity is associated with an increased risk of mortality and exacerbations30,31; (c) when compared to healthy individuals of the same age, COPD patients have a lower level of PA32; (d) the comorbidities that COPD patients may present also improve with regular PA33; (e) physical inactivity is associated with progressive intolerance of exercise and muscular involvement34; (f) low levels of PA occur even in patients with mild COPD, suggesting that early intervention is needed to reduce the risk of future comorbidities and, possibly, disease progression35,36; (g) PA in patients with COPD depends on psychological, behavioral, social, environmental, and cultural factors,27 and (h) the health professional must be convinced that the prescription of PA should form part of the treatment of every COPD patient.37
To date, no specific recommendations have been established with respect to the level of PA that COPD patients should maintain. However, general recommendations on the benefits of performing moderate PA for at least 30min a day, 5 days a week (or the equivalent of 1000kcal/week), such as those of American College of Sports Medicine,38 are generally accepted.
In the Phenotype and Course of Chronic Obstructive Pulmonary Disease (PAC-COPD) cohort, subjects with severe to very severe COPD performed their daily activities over shorter and fewer periods than patients with mild to moderate stages of the disease.39 This pattern should be taken into account when PA is being recommended and prescribed in more severe patients, since these patients must perform short sessions of PA several times a day, instead of longer, uninterrupted sessions of 20–30min.
The prescription of PA is complex because it involves a combination of individual, socio-cultural and environmental factors that must be taken into account.30,31 Consequently, and in view of the lack of evidence and consensus in prescription criteria and in strategies to increase the PA in COPD patients, strategies adapted to everyday life should be proposed and agreed upon with the patient.
Table 2 describes randomized clinical trials in which a PA intervention was prescribed, and the tool used to measure PA in COPD patients. In a recent systematic review conducted by Mantoani et al.,40 the authors concluded that interventions with PR programs of longer than 12 weeks’ duration have a positive impact on increasing PA.
Randomized Controlled Trials Associated With Different Strategies to Increase the Level of Physical Activity, Classified by Evaluation Tool (Questionnaire or Diary, Accelerometers, and Pedometers), Listed by Author.
| Authors | Intervention | Instrument of Evaluation |
|---|---|---|
| PA questionnaires/diaries | ||
| Benstrup (1997) Faager (2004) Ries (1995) | PR program | Questionnaire on activities of daily living, specific score for COPD Stanford Health Assessment Questionnaire Adaptation of self-efficacy questionnaire |
| Berry (2010) | PR program+self-management | CHAMPS Physical Activity Questionnaire for Older Adults questionnaire |
| Duiverman (2011) | PR program+night-time CPAP | Groningen Activity Questionnaire and Restriction Scale |
| Engström (1999) | PR program+walking program | Sickness Impact Profile Questionnaire |
| Faulkner (2010) | PR program+PA counseling | Stanford 7-day physical activity recall questionnaire |
| Kesten (2008) Shioya (2008) | Bronchodilator | Non-validated questionnaire London Chest Activities of Daily Living Scale Questionnaire |
| Mohammadi (2013) | Home PR program | Barthel index questionnaire |
| Nield (2007) | Breathing exercises | Human Activity Profile Questionnaire |
| Pleguezuelos (2013) | PA counseling | Daily walking diary |
| Steele (2008) | Walking program+PA counseling | Walking Self-Efficacy questionnaire |
| Vergeret (1989) | Oxygen therapy | Non-validated questionnaire |
| Weekes (2009) | Nutritional supplements | Townsend score questionnaire |
| Yohannes (2003) | Walking program+oxygen therapy | Barthel index questionnaire |
| PA monitors: accelerometers | ||
| Borges (2014) Larson (2014) | Resistance training | DynaPort accelerometer Actigraph 7164 accelerometer |
| Breyer (2010) Pomidori (2012) | Walking program | DynaPort accelerometer SenseWear Armband Pro3 accelerometer |
| Casaburi (2012) Sandland (2008) | Oxygen therapy | RT3 accelerometer Gaehwiler Z80 accelerometer |
| Dal Negro (2010), Dal Negro (2012) Goris (2003) | Nutritional supplements | SenseWear Armband Pro3 accelerometer Tracmor accelerometer |
| Nguyen (2009) | PA counseling | StepWatch 3 accelerometer |
| Probst (2011) Sewell (2005), Sewell (2010) | PR program | DynaPort accelerometer and SenseWear Armband Pro3 Gaehwiler Z80 accelerometer |
| Steele (2008) | Walking program+PA counseling | RT3 accelerometer |
| Troosters (2014), Watz (2014) | Bronchodilator | SenseWear Armband Pro3 accelerometer |
| PA monitors: accelerometers | ||
| Altenburg (2015), Hospes (2009), Tabak (2013) Mendoza (2015) | PA counseling | Yamax Digi Walker SW-200 pedometer Tanita PD724 pedometer |
| Bauldoff (2002) | Walking program | Sportline 342 pedometer + PA diary |
| de Blok (2006) | PR program+PA counseling | Yamax Digi Walker SW-200 pedometer |
| Duiverman (2008) | PR program+night-time CPAP | Yamax Digi Walker SW-200 pedometer |
| Effing (2011) | PR plus self-management | Yamax Digi Walker SW-200 pedometer |
PA: physical activity; CPAP: continuous positive airway pressure; PR: pulmonary rehabilitation.
Adapted from Mantoani et al.40
Other more specific alternatives, such as dietary intervention in patients with cachexia and nocturnal non-invasive ventilation, along with exercise in subjects with hypercapnia, appear to improve PA levels in these patients.40 Long-term oxygen therapy (≥8 weeks) and electrical muscle stimulation do not have a significant impact on PA levels, but could be used adjunctively with other interventions. Finally, bronchodilators may increase activity levels in the daily life of subjects with COPD.40
It is important to remember that behaviors associated with PA are complex, and as such, difficult to modify. Changing the attitude of the COPD patient toward PA requires an interdisciplinary approach that involves medical, social, and behavioral sciences, and interventions should be based on theoretical models of behavior change.27 The latest ERS guidelines on PA in COPD emphasize that social support and the use of specific techniques for behavior modification are essential for achieving better contact with patients and higher motivation for change, and as such, are core elements for making behavioral interventions more effective.27
In practice, COPD patients should be encouraged to increase their level of PA and they must be clearly informed of the therapeutic benefits of activity.37 Strategies aimed at increasing motivation for performing PA, and therefore, adherence to PA programs should include specific, measurable, achievable, realistic objectives, set down in writing, ensuring that the patient plays an active role in the control of their disease.27,37
Self-Care StrategiesCOPD patients should have access to educational programs that teach the skills necessary for management of their disease. This includes activities aimed at maintaining and improve their health, well-being, and ensuring appropriate therapeutic adherence and compliance. The most important aspects are41:
- –
Protection from harmful substances in the environment (masks).
- –
Maintaining an optimal environment in the home.
- –
Proper adherence to pharmacological and non-pharmacological treatment.
- –
Regular exercise.
- –
Good sleep hygiene: establish routines for going to bed, getting up and resting. Avoid the ingestion of caffeine and alcohol.
- –
Adapt personal hygiene routines to avoid problems with dyspnea.
- –
Wear loose clothing, appropriate for the season.
- –
Keep enjoying recreational and social activities and taking an interest.
Limited information is available on the implementation of the chronic care model in patients with chronic respiratory diseases such as COPD. Adams et al.6 found that patients with COPD who received interventions with 2 or more elements required fewer hospital admissions for acute COPD exacerbations and shorter hospital stays.
Living with a chronic illness is difficult, and even more so when self-care is complex. Achieving good treatment adherence is one of the most important challenges and a key therapeutic factor, although it is a complex process that is influenced by multiple factors associated with the patient, the professional, the health system, the disease, and the drugs themselves.42
The aim of TE is to help patients acquire the necessary resources to manage their lives as well as possible, and to be informed and to share decision-making regarding their health and their disease with their attending healthcare professionals.42,43
The WHO advises that all patients with chronic disease should have access to structured TE programs and an individualized treatment plan, and that it is essential that healthcare professionals acquire the knowledge and skills required to implement such programs, although the level of training in this field is still limited.43
To plan an individualized TE plan, patient priorities, treatment complexity, and healthcare models must be taken into account, and the patient must be evaluated according to an overall assessment comprising 5 dimensions: healthcare model, socioeconomic factors, treatment-related factors, patient-related factors, and finally, disease-related factors.43
Therapeutic adherence in COPD is low, leading to increased rates of exacerbations, hospitalizations, and death.44 TE alone has no impact on the use of healthcare resources or on the patient's functional status, but results are positive if it is accompanied by other interventions.45
A Cochrane review46 found that self-care interventions in COPD patients are associated with improved quality of life, fewer hospital admissions, and an improved perception of dyspnea. However, differing interventions, study populations and follow-up periods, and the range of outcome measures make it difficult to formulate clear recommendations with regard to the most effective form and content of self-care in COPD, and as such TE.
The latest ERS expert consensus47 concludes that a self-care program should be structured, personalized, and multicomponent, in order to motivate, involve and support patients in adopting healthy behaviors and developing skills to help them better manage their disease and comply with treatment.
Published studies do not provide conclusive data for designing the optimal TE program in COPD, although a recent meta-analysis showed that longer self-care interventions reduce hospitalizations for all causes.48
The GesEPOC working group proposes the essential components of a TE program in COPD and the elements that must be evaluated to determine the degree of patient adherence (Table 3). Support materials used for the development of educational interventions should be based on clinical guidelines and must be validated.49,50
Elements that Should be Included in a Therapeutic Education Program.
| Understanding of the disease |
| Ensure correct administration of treatment |
| Know how to promptly identify and react to exacerbations |
| Incorporate and maintain a lifestyle with the recommended changes |
| Avoid risk behaviors |
| Attend scheduled visits |
| Awareness and control of comorbidities |
| Maintain a proactive attitude |
Maintaining an optimal nutritional status is crucial in patients with respiratory diseases. The effects of malnutrition on the respiratory system have an impact on respiratory muscles, lung parenchyma, and the immune system.51,52
Evaluation of a patient's nutritional status is essential for early detection of subjects at a higher risk of malnutrition and to establish the required level of nutritional support. Evaluation of these patients must include a dietary study, an anthropometric study (weight, height, body mass index, skinfold measurement), and hematological, biochemical, and immunological tests.53 Techniques such as electrical bioimpedance analysis are very useful for determining body composition. Strategies listed in Table 4 should be followed in patients with and without nutritional deficits.
Nutritional Strategies.
| Meals should be taken 5–6 times a day, in small portions, eating slowly and chewing well |
| Eat at least 5 servings (total 600g) of fruit and vegetables a day |
| Eat fish 2–3 times a week, with at least 2 servings of oily fish (sardines, anchovies, tuna, mackerel, salmon) |
| Reduce consumption of animal fat |
| Preferably use virgin olive oil for cooking and dressings |
| Do not eat very cold or very hot food, since this can cause coughing or choking |
| Drink plenty of fluids (approximately 1.5–2l), unless contraindicated |
| The consumption of alcoholic beverages is not recommended due to their known risk of causing other diseases |
Obesity is another important factor that is increasingly encountered in COPD. Obese patients may have limited exercise capacity, respiratory restriction, and worse dyspnea. Dietary advice is essential in these cases. It is important that dysphagia is evaluated in patients with chronic respiratory disease, in order to minimize complications.54,55
In this article, we have discussed the most important points that must be taken into consideration in the non-pharmacological treatment of COPD. Our conclusions are summarized in Table 5. This article highlights the importance of non-pharmacological treatment in the correct management of COPD patients.
Key Points in the Non-pharmacological Treatment of the COPD Patient.
| Pulmonary rehabilitation improves dyspnea, exercise capacity, and quality of life |
| Pulmonary rehabilitation programs are safe |
| Currently, pulmonary rehabilitation should be avoided during hospital admission |
| The implementation of home maintenance programs is a valid alternative to rehabilitation in the hospital, starting at the initial stages of the disease |
| Avoiding a sedentary lifestyle and promoting activity and daily physical exercise is beneficial for the COPD patient, and should be widely recommended |
| The prescription of PA should be individualized and should take into account exercise capacity, comorbidities or disability, family and social environment, and behavioral and cultural factors of the COPD patient |
| Integrated care and active involvement of patients are fundamental in the management of chronic diseases |
| All chronic patients should have access to structured therapeutic education programs and an individualized treatment plan |
| Self-care programs should be structured, personalized, and involve multiple components |
| Assessment of the patient's nutritional status is essential to avoid malnutrition and obesity |
Marc Miravitlles has received speaker fees from Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, CSL Behring, Grifols and Novartis, consulting fees from Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, CSL Behring, Laboratorios Esteve, Mereo Biopharma, Verona Pharma, pH Pharma, Novartis and Grifols and research grants from GlaxoSmithKline and Grifols, all outside the submitted work.
The rest authors state that they have no conflict of interests.
ANNEX GesEPOC OrganizationCoordinator: Marc Miravitlles, Sociedad Española de Neumología y Cirugía Torácica (SEPAR). Working group member: Myriam Calle, Borja García-Cosío, Juan Antonio Riesco, Eusebi Chiner, Ciro Casanova and Juan José Soler-Cataluña (SEPAR). Joan B. Soriano (SEPAR-Epidemiology). Julio Ancochea, coordinador científico de la estrategia en EPOC del SNS (SEPAR). Pere Almagro, Sociedad Española de Medicina Interna (SEMI). Elena Gimeno (SEPAR-Physiotherapy). Eulogio Pleguezuelos, Sociedad Española de Rehabilitación y Medicina Física y Sociedad de Rehabilitación Cardio-Respiratoria (SERMEF/SORECAR). José Antonio Quintano, Sociedad Española de Médicos de Atención Primaria (SEMERGEN). Juan Antonio Trigueros, Sociedad Española de Médicos Generales y de Familia (SEMG). Jesús Molina, Sociedad Española de Medicina de Familia y Comunitaria (SemFYC). Pere Simonet, Sociedad de Respiratorio en Atención Primaria (GRAP). Pascual Piñera and Adolfo Simón, Sociedad Española de Medicina de Urgencias y Emergencias (SEMES). Carme Hernández and Carmen Mata (SEPAR-Enfermería). Leopoldo Palacios and Carlos Verdejo, Sociedad Española de Geriatría y Gerontología (SEGG). María Emilia Carretero Díaz, Foro Español de Pacientes (FEP). David Rigau and Ena Pery Niño de Guzman Quispe, Centro Cochrane Iberoamericano (Barcelona).
Please cite this article as: Pleguezuelos E, Gimeno-Santos E, Hernández C, del Carmen Mata M, Palacios L, Piñera P, et al. Recomendaciones sobre tratamiento no farmacológico en la enfermedad pulmonar obstructiva crónica de la Guía española de la EPOC (GesEPOC 2017). Arch Bronconeumol. 2018;54:568–575.