The objective of this study was to analyze the clinical characteristics of COPD patients attending general respiratory clinics, adherence to clinical guidelines, classification accuracy, and therapeutic management.
MethodMulticenter, cross-sectional study, with the primary objective of describing the number, intensity, and variability of symptoms in COPD patients. Data were collected in 2015 by 300 pulmonologists who evaluated a total of 3010 patients, of which 2669 (88.6%) were eligible for analysis.
ResultsA total of 22% were active smokers, notably 21% and 17% of GOLD groups C and D, and 17% and 19% of exacerbators in the GesEPOC classification; 62.3% had associated comorbidities. The diagnostic process was characterized by limited use of tests such as diffusion capacity or lung volumes, even in the more severe GOLD groups or the GesEPOC exacerbator phenotypes. The use of multidimensional scales, such as BODE (12%), and specific rehabilitation protocols was also rare. Treatment was based on different combinations of bronchodilators and inhaled corticosteroids. Methylxanthines were used in very few patients (7%).
ConclusionsA large proportion of COPD patients seen in respiratory clinics have non-complex disease and pulmonology tests are rarely performed. Our study confirms a slight change in treatments and the frequent association with comorbidities that can modify the clinical presentation of the patient.
El objetivo de este estudio ha sido analizar las características clínicas de los pacientes con EPOC vistos en las consultas generales de neumología, el seguimiento de las guías clínicas y la adecuación en la categorización y en el manejo terapéutico.
MétodoEstudio multicéntrico, transversal, cuyo objetivo primario es describir el número, intensidad y variabilidad de los síntomas en los pacientes con EPOC. La recogida de los datos fue realizada en 2015 por 300 neumólogos que evaluaron un total de 3.010 pacientes, de los cuales 2.669 (88,6%) fueron válidos para el análisis.
ResultadosEl 22% eran fumadores activos, destacando un 21 y 17% en los grupos C y D de GOLD y un 17 y 19% en los pacientes exacerbadores de GesEPOC. El 62,3% presentaba comorbilidades asociadas. En el proceso diagnóstico destaca el bajo uso de pruebas como el test de difusión o la medición de volúmenes pulmonares, incluso en los grupos más graves de GOLD o en los fenotipos exacerbadores de GesEPOC. También es minoritario el uso de escalas multidimensionales como BODE (12%) o programas específicos de rehabilitación. El tratamiento se basa en el uso de broncodilatadores y corticoides inhalados, en diferentes combinaciones. El uso de metilxantinas fue minoritario (7%).
ConclusionesEn consultas de neumología existe una elevada proporción de pacientes EPOC con baja complejidad y un escaso uso de pruebas neumológicas. Se constata un leve cambio en las pautas terapéuticas y la frecuente asociación con comorbilidades que pueden modificar la presentación clínica del paciente.
COPD is one of the leading causes of morbidity and mortality in our setting. Although it has an irreversible component, we now know that the right diagnosis and appropriate treatment can alter the natural history of the disease. Indeed, recent data have confirmed a reduction in hospital admissions for acute exacerbations and mortality due to COPD.1–3 Moreover, lung function has been seen to stabilize in a high proportion of patients, possibly because of the availability of better treatments, shattering the myth that COPD progression is inevitable.4
Despite these favorable data, patient management often fails to comply with clinical guideline recommendations, prompting concerns regarding quality in the diagnosis and treatment of COPD.5,6 The clinical presentation of COPD is heterogeneous, so clinical guidelines, such as GesEPOC,7 propose that patients are classified according to phenotypes. This approach is complicated further by the presence of comorbidities that affect the clinical expression of the disease. The consequence of this complexity is that situations may arise that generate significant variability in the management of COPD and non-compliance with recommendations that are based on the best available evidence.
Although COPD patients are usually managed by several specialists, in most health settings, the pulmonologist is usually responsible for maintaining adherence to the clinical management guidelines. The key to understanding the characteristics of COPD in a real-world setting, and to identifying areas for improvement that will assist in the implementation of strategic plans in different health areas, lies in an analysis of the tools used by specialists in the diagnostic process, the clinical characteristics of patients according to the current classification criteria, and the treatment provided in this care setting.
The objective of this study was to analyze the clinical characteristics of COPD patients attending respiratory clinics, adherence to clinical guidelines in the diagnostic process, and the adequacy of classifications and therapeutic management.
MethodWe present data from the ConEPOC study, a multicenter, cross-sectional study performed in respiratory clinics, the primary objective of which was to describe the number, intensity, and variability of symptoms in COPD patients.8 Data were collected in 2015 by 300 pulmonologists who evaluated a total of 3010 patients, of which 2669 (88.6%) were eligible for analysis.
The participating investigators recorded previously defined current and retrospective data in the electronic case report form (eCRF). These data were obtained from the medical histories of patients and from the patients themselves in the study visit.
To be included in the study, all patients had to be older than 40 years, current or former smokers with a history of at least 10 pack-years, and with a spirometry and bronchodilator test with a FEV1/FVC ratio less than 70%. All patients included in the study were outpatients, and all were stabilized. Exclusion criteria were the existence of other chronic lung disorders, including a recent diagnosis of asthma, or an exacerbation within 1 month prior to the visit. Patients were selected sequentially, with a total of 10 patients per center. In addition to collecting pre-diagnosis, staging and treatment data, investigators performed their own classification and phenotyping, if this had not been performed previously. Patients were classified according to the GOLD 2013 recommendations,9 which include 4 groups (A, B, C, D) based on symptoms, exacerbations during the year prior to the visit, and lung function. Patients were also classified according to their clinical profile using the GesEPOC recommendations: asthma-COPD overlap (ACO), non-exacerbator, exacerbator without bronchitis (emphysema), and exacerbator with bronchitis. Symptoms were collected using questionnaires and validated scales. Dyspnea was determined using the modified Medical Research Council (mMRC) scale. The impact of COPD on the wellbeing and daily life of the patient was evaluated with the COPD Assessment Test (CAT) questionnaire. The CASIS sleep questionnaire,10,11 which takes into account the effect of respiratory problems due to COPD/asthma on sleep during the last 7 days, was used to analyze the impact of night-time symptoms. Symptoms at night and in the early hours of the day were specifically analyzed with the Night-time and Early Morning Symptoms of COPD Instruments (NiSCI and EM-SCI), which are scored from 0 (no symptoms) to 4 (very severe symptoms).12–14
To evaluate the variability of symptoms, patients had to complete a questionnaire that included daily symptoms over a 7-day period. The patient was considered to have variable symptoms if a symptom appeared or resolved at any time during this follow-up period, and symptom intensity (morning/night) was considered to be variable when 2 or more symptoms progressed from mild/moderate to severe/very severe during this period. Overall variability was calculated as the sum of the patients who had variable symptoms and/or intensity.8 The criterion used to evaluate exacerbations was an increase in respiratory symptoms that required a change in the patient's usual medication at the discretion of the investigator during the year prior to inclusion in the study. Information was collected retrospectively, and exacerbations that required hospitalization were classified as severe and those that did not were classified as moderate.
The sample size was initially estimated at 2400 patients, with a maximum standard error of 2% for changes in the main variable, the prevalence and severity of night-time and daytime COPD symptoms. This sample size and the wide geographical distribution of participating hospitals, which encompassed the whole of Spain, ensures an appropriate representation of the management of COPD in specialized care in Spain.
For the analysis, each type of variable was compared using the appropriate tests. The Chi-squared test was used for qualitative variables; for small samples (more than 20% of contingency table cells with an expected frequency of less than 5), the Fisher test or Yates correction were used, according to the number of categories. For continuous variables, goodness-of-fit for a normal distribution was tested; in cases that met the assumptions of normality, we used parametric tests (Student's t-test or ANOVA), and appropriate non-parametric tests were used if data distributions were not normal (Mann–Whitney U test or Kruskal–Wallis test). A 2-tailed level of significance of 0.05 was used for all tests. All analyses were performed using the SPSS statistical package, version 23.0. This study was designed and performed in accordance with the Guidelines for Good Pharmacoepidemiology Practices (GPP) of the International Society of Pharmacoepidemiology (http://www.pharmacoepi.org/resources/guidelines_08027.cfm), the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (http://www.strobe-statement.org/index.php?id=available-checklists), and the ethical principles set down in the Declaration of Helsinki (http://www.wma.net/en/30publications/10policies/b3/index.html).
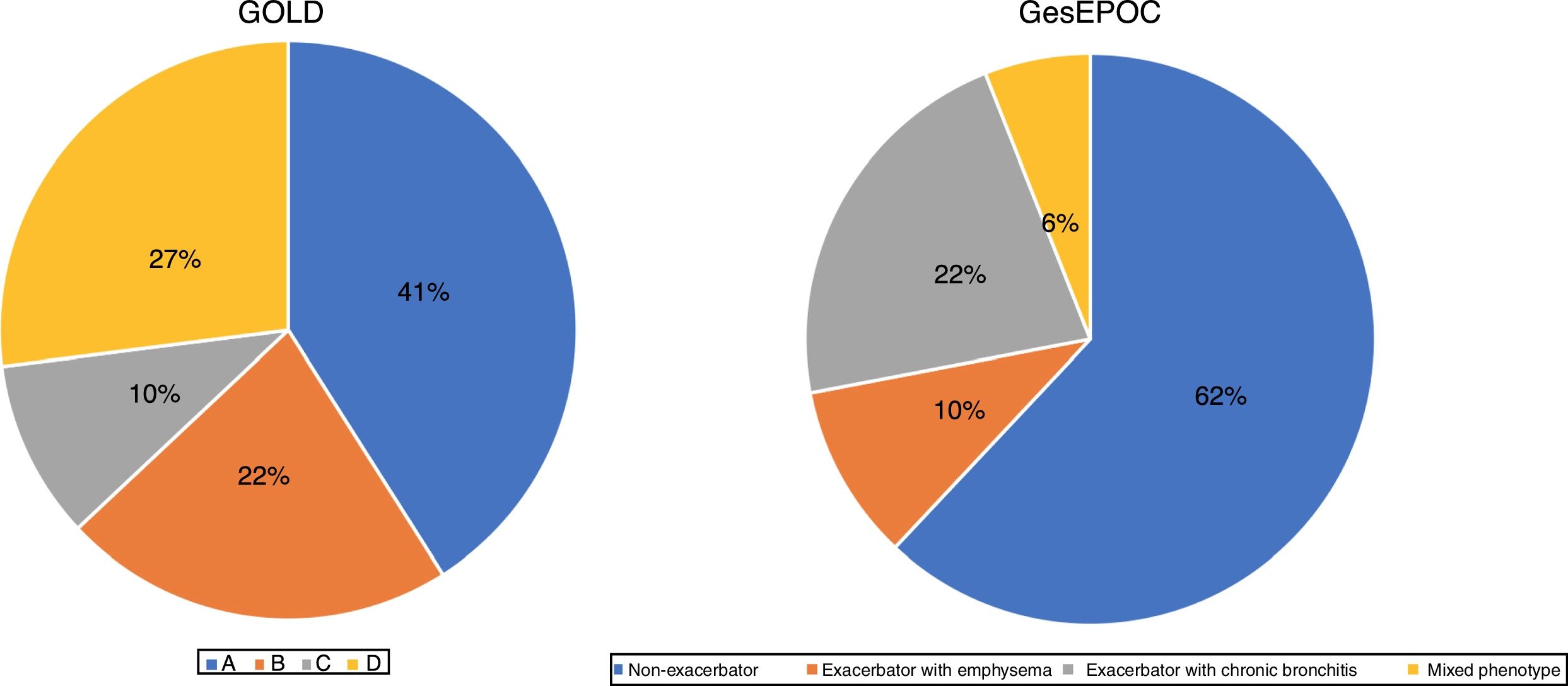
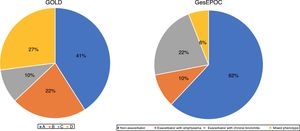
ResultsOf a total of 3010 patients, 2669 (88.6%) were eligible for analysis. The remaining patients were excluded due to incomplete questionnaire data. In 46.9% of patients, a GesEPOC phenotype had been established before the study, in 94.4% of cases by a pulmonologist. In the remaining cases, the phenotype was established at the time of the visit, definitively in 80% of the cases. GOLD classification was available for 38.5% of the patients. The final classification was definitive in 73% of the patients. Ultimately, 2629 patients were assigned to a group according to the GesEPOC and GOLD criteria (Fig. 1). The GOLD classification was made using the CAT questionnaire in 32% of patients in group A, 48% in group B, 25% in group C, and 55% in group D, and by mMRC in 28%, 37%, 22% and 45%, respectively. The rest fulfilled both criteria.
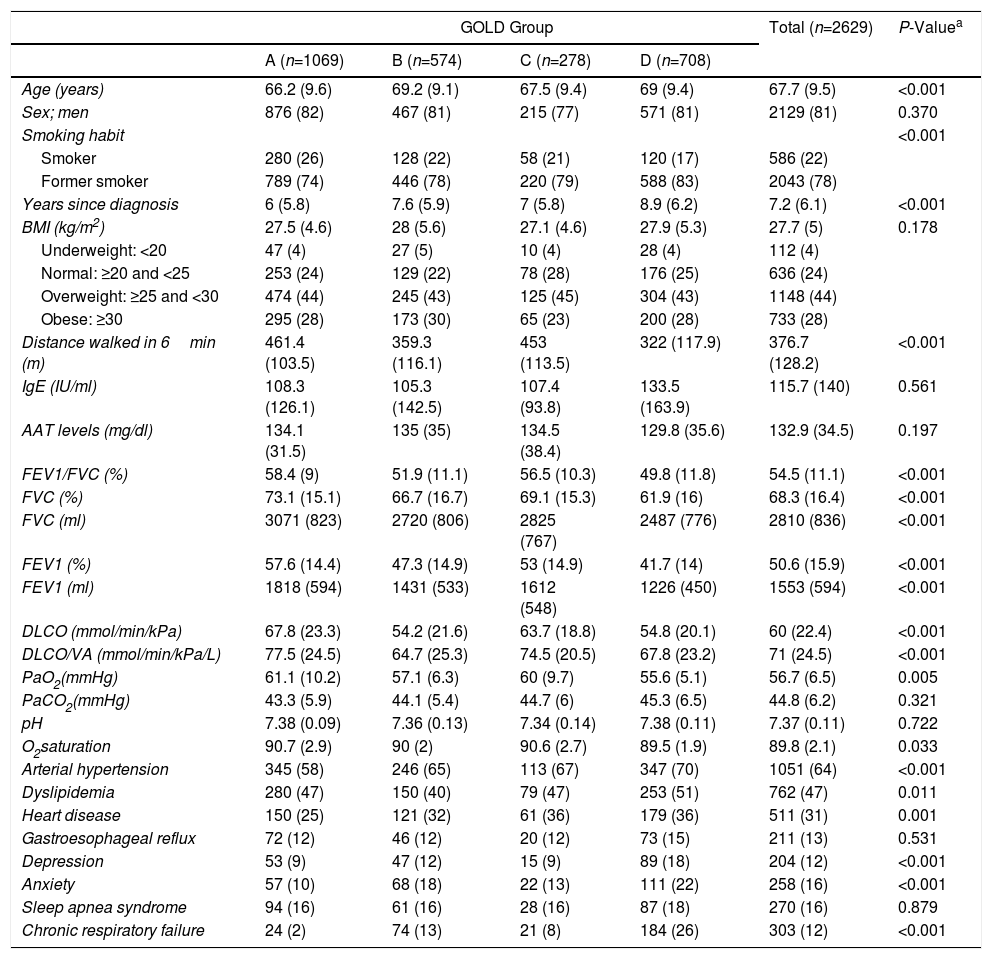
Table 1 lists demographic, anthropometric, analytical, functional data, and the most significant comorbidities of the study population, with a separate analysis of each GOLD (Table 1a) and GesEPOC (Table 1b) group. In total, 81% of patients were men, with a mean age of 68 years. A total of 22% were active smokers, notably 21% and 17% of GOLD groups C and D, and 17% and 19% of exacerbators in the GesEPOC classification. The majority were of normal weight or overweight (BMI of 27.3), with a mean FEV1 of around 50%. Only 4% of patients had BMI below 20.
Baseline Characteristics by GOLD Group.
| GOLD Group | Total (n=2629) | P-Valuea | ||||
|---|---|---|---|---|---|---|
| A (n=1069) | B (n=574) | C (n=278) | D (n=708) | |||
| Age (years) | 66.2 (9.6) | 69.2 (9.1) | 67.5 (9.4) | 69 (9.4) | 67.7 (9.5) | <0.001 |
| Sex; men | 876 (82) | 467 (81) | 215 (77) | 571 (81) | 2129 (81) | 0.370 |
| Smoking habit | <0.001 | |||||
| Smoker | 280 (26) | 128 (22) | 58 (21) | 120 (17) | 586 (22) | |
| Former smoker | 789 (74) | 446 (78) | 220 (79) | 588 (83) | 2043 (78) | |
| Years since diagnosis | 6 (5.8) | 7.6 (5.9) | 7 (5.8) | 8.9 (6.2) | 7.2 (6.1) | <0.001 |
| BMI (kg/m2) | 27.5 (4.6) | 28 (5.6) | 27.1 (4.6) | 27.9 (5.3) | 27.7 (5) | 0.178 |
| Underweight: <20 | 47 (4) | 27 (5) | 10 (4) | 28 (4) | 112 (4) | |
| Normal: ≥20 and <25 | 253 (24) | 129 (22) | 78 (28) | 176 (25) | 636 (24) | |
| Overweight: ≥25 and <30 | 474 (44) | 245 (43) | 125 (45) | 304 (43) | 1148 (44) | |
| Obese: ≥30 | 295 (28) | 173 (30) | 65 (23) | 200 (28) | 733 (28) | |
| Distance walked in 6min (m) | 461.4 (103.5) | 359.3 (116.1) | 453 (113.5) | 322 (117.9) | 376.7 (128.2) | <0.001 |
| IgE (IU/ml) | 108.3 (126.1) | 105.3 (142.5) | 107.4 (93.8) | 133.5 (163.9) | 115.7 (140) | 0.561 |
| AAT levels (mg/dl) | 134.1 (31.5) | 135 (35) | 134.5 (38.4) | 129.8 (35.6) | 132.9 (34.5) | 0.197 |
| FEV1/FVC (%) | 58.4 (9) | 51.9 (11.1) | 56.5 (10.3) | 49.8 (11.8) | 54.5 (11.1) | <0.001 |
| FVC (%) | 73.1 (15.1) | 66.7 (16.7) | 69.1 (15.3) | 61.9 (16) | 68.3 (16.4) | <0.001 |
| FVC (ml) | 3071 (823) | 2720 (806) | 2825 (767) | 2487 (776) | 2810 (836) | <0.001 |
| FEV1 (%) | 57.6 (14.4) | 47.3 (14.9) | 53 (14.9) | 41.7 (14) | 50.6 (15.9) | <0.001 |
| FEV1 (ml) | 1818 (594) | 1431 (533) | 1612 (548) | 1226 (450) | 1553 (594) | <0.001 |
| DLCO (mmol/min/kPa) | 67.8 (23.3) | 54.2 (21.6) | 63.7 (18.8) | 54.8 (20.1) | 60 (22.4) | <0.001 |
| DLCO/VA (mmol/min/kPa/L) | 77.5 (24.5) | 64.7 (25.3) | 74.5 (20.5) | 67.8 (23.2) | 71 (24.5) | <0.001 |
| PaO2(mmHg) | 61.1 (10.2) | 57.1 (6.3) | 60 (9.7) | 55.6 (5.1) | 56.7 (6.5) | 0.005 |
| PaCO2(mmHg) | 43.3 (5.9) | 44.1 (5.4) | 44.7 (6) | 45.3 (6.5) | 44.8 (6.2) | 0.321 |
| pH | 7.38 (0.09) | 7.36 (0.13) | 7.34 (0.14) | 7.38 (0.11) | 7.37 (0.11) | 0.722 |
| O2saturation | 90.7 (2.9) | 90 (2) | 90.6 (2.7) | 89.5 (1.9) | 89.8 (2.1) | 0.033 |
| Arterial hypertension | 345 (58) | 246 (65) | 113 (67) | 347 (70) | 1051 (64) | <0.001 |
| Dyslipidemia | 280 (47) | 150 (40) | 79 (47) | 253 (51) | 762 (47) | 0.011 |
| Heart disease | 150 (25) | 121 (32) | 61 (36) | 179 (36) | 511 (31) | 0.001 |
| Gastroesophageal reflux | 72 (12) | 46 (12) | 20 (12) | 73 (15) | 211 (13) | 0.531 |
| Depression | 53 (9) | 47 (12) | 15 (9) | 89 (18) | 204 (12) | <0.001 |
| Anxiety | 57 (10) | 68 (18) | 22 (13) | 111 (22) | 258 (16) | <0.001 |
| Sleep apnea syndrome | 94 (16) | 61 (16) | 28 (16) | 87 (18) | 270 (16) | 0.879 |
| Chronic respiratory failure | 24 (2) | 74 (13) | 21 (8) | 184 (26) | 303 (12) | <0.001 |
| Table 1b: Baseline Characteristics by GesEPOC Phenotype | ||||||
|---|---|---|---|---|---|---|
| GesEPOC Phenotype | Total (n=2628) | P-Valuea | ||||
| ACO (n=169) | Non-Exacerbator (n=1639) | Exacerbator with Bronchitis (n=567) | Exacerbator without Bronchitis (n=253) | |||
| Age (years) | 66.1 (9.6) | 67.3 (9.4) | 69.3 (9.7) | 68.3 (9.1) | 67.7 (9.5) | <0.001 |
| Sex; men | 121 (72) | 1338 (82) | 470 (83) | 200 (79) | 2129 (81) | 0.007 |
| Smoking habit | <0.001 | |||||
| Smoker | 38 (22) | 405 (25) | 94 (17) | 47 (19) | 584 (22) | |
| Former smoker | 131 (78) | 1234 (75) | 473 (83) | 206 (81) | 2044 (78) | |
| Years since diagnosis | 6.8 (5.8) | 6.6 (5.9) | 9 (6.1) | 8.2 (6.4) | 7.2 (6.1) | <0.001 |
| BMI (kg/m2) | 28.2 (4.8) | 27.7 (5) | 27.8 (5.4) | 27.2 (4.9) | 27.7 (5.1) | 0.116 |
| Underweight: <20 | 2 (1) | 75 (5) | 24 (4) | 11 (4) | 112 (4) | 0.147 |
| Normal: ≥ 20 and <25 | 33 (19) | 388 (24) | 147 (26) | 70 (28) | 638 (24) | |
| Overweight: ≥ 25 and <30 | 84 (50) | 710 (43) | 233 (41) | 115 (45) | 1142 (43) | |
| Obese: ≥ 30 | 50 (30) | 466 (28) | 163 (29) | 57 (23) | 736 (28) | |
| Distance walked in 6min (m) | 409.8 (94.3) | 406.4 (123.1) | 320 (119.9) | 366.1 (138.3) | 376.5 (128.2) | <0.001 |
| IgE (IU/ml) | 185.7 (186.7) | 91.2 (110.6) | 107.8 (138.8) | 105.5 (113.5) | 115.4 (139.7) | <0.001 |
| AAT levels (mg/dl) | 126.1 (23.1) | 135.5 (33.4) | 127.4 (35.4) | 139.4 (42.7) | 133 (34.6) | 0.016 |
| FEV1/FVC (%) | 56.2 (10.3) | 55.8 (10.4) | 51.2 (11.7) | 52 (12.2) | 54.5 (11) | <0.001 |
| FVC (%) | 70.8 (14.9) | 70.6 (16) | 62.3 (16.1) | 65.3 (16) | 68.3 (16.4) | <0.001 |
| FVC (ml) | 2850 (864) | 2934 (830) | 2522 (796) | 2668 (759) | 2813 (835) | <0.001 |
| FEV1 (%) | 54.5 (15.2) | 53.5 (15.4) | 43.1 (15) | 46.2 (14.6) | 50.6 (15.9) | <0.001 |
| FEV1 (ml) | 1639 (615) | 1666 (598) | 1285 (507) | 1391 (489) | 1556 (593) | <0.001 |
| DLCO (mmol/min/kPa) | 71.8 (20.1) | 61.2 (23.3) | 54.9 (20.1) | 56.1 (19.2) | 60 (22.4) | <0.001 |
| DLCO/VA (mmol/min/kPa/L) | 84 (21.8) | 71.2 (25.2) | 67.6 (24.1) | 68.4 (20.1) | 71 (24.5) | 0.001 |
| PaO2(mmHg) | 58 (9.4) | 58.1 (7.6) | 56.1 (5.7) | 55.1 (4) | 56.8 (6.5) | 0.051 |
| PaCO2(mmHg) | 45.8 (5.3) | 43.7 (5.5) | 45.8 (6.4) | 44.3 (7) | 44.9 (6.2) | 0.016 |
| pH | 7.4 (0.03) | 7.36 (0.13) | 7.37 (0.11) | 7.39 (0.08) | 7.37 (0.11) | 0.498 |
| O2saturation | 90.1 (2.4) | 90.2 (2.3) | 89.7 (2) | 89 (1.6) | 89.8 (2.1) | 0.018 |
| Arterial hypertension | 64 (59) | 601 (61) | 269 (72) | 121 (70) | 1055 (64) | <0.001 |
| Dyslipidemia | 47 (43) | 437 (44) | 195 (52) | 82 (47) | 761 (46) | 0.064 |
| Heart disease | 31 (28) | 275 (28) | 141 (38) | 65 (37) | 512 (31) | 0.001 |
| Gastroesophageal reflux | 14 (13) | 123 (13) | 58 (16) | 20 (11) | 215 (13) | 0.447 |
| Depression | 13 (12) | 105 (11) | 58 (16) | 30 (17) | 206 (13) | 0.021 |
| Anxiety | 17 (16) | 131 (13) | 72 (19) | 40 (23) | 260 (16) | 0.002 |
| Sleep apnea syndrome | 12 (11) | 157 (16) | 72 (19) | 31 (18) | 272 (17) | 0.178 |
| Chronic respiratory failure | 15 (9) | 108 (7) | 132 (23) | 44 (17) | 299 (11) | <0.001 |
Data expressed as mean (SD) or n (%).
DLCO: diffusing capacity of carbon monoxide; DLCO/VA: diffusing capacity of carbon monoxide/alveolar volume.
In total, 62.3% of the patients had a significant medical history and/or concomitant diseases. The most common concomitant disease was arterial hypertension, present in 40% of the overall series, followed by dyslipidemia (29%). Table 1 shows the comorbidities associated with COPD that can directly participate in modulating the clinical expression of the patient. The prevalence of concomitant heart disorders was high (31%), particularly in the more severe GOLD groups (P<0.001) and GesEPOC exacerbator phenotypes (P<0.001).
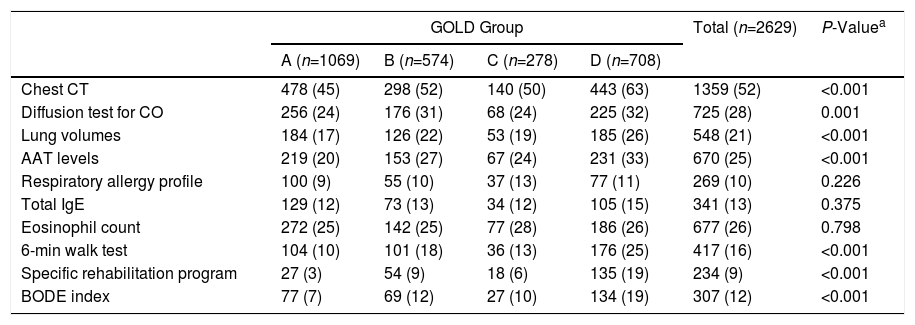
Table 2 lists the tests performed in pulmonology clinics during the COPD diagnostic process. The diagnostic process was characterized by the limited use of basic tests such as diffusion or lung volumes, even in the more severe GOLD groups or the GesEPOC exacerbator phenotypes. The use of multidimensional scales, such as BODE (12%), and specific rehabilitation protocols was also rare (9%), even in severe patients and/or exacerbators. Alpha-1 antitrypsin (AAT) determinations, which should be mandatory at least once in all patients, were carried out only in 25%.
Tests Performed by GOLD Group.
| GOLD Group | Total (n=2629) | P-Valuea | ||||
|---|---|---|---|---|---|---|
| A (n=1069) | B (n=574) | C (n=278) | D (n=708) | |||
| Chest CT | 478 (45) | 298 (52) | 140 (50) | 443 (63) | 1359 (52) | <0.001 |
| Diffusion test for CO | 256 (24) | 176 (31) | 68 (24) | 225 (32) | 725 (28) | 0.001 |
| Lung volumes | 184 (17) | 126 (22) | 53 (19) | 185 (26) | 548 (21) | <0.001 |
| AAT levels | 219 (20) | 153 (27) | 67 (24) | 231 (33) | 670 (25) | <0.001 |
| Respiratory allergy profile | 100 (9) | 55 (10) | 37 (13) | 77 (11) | 269 (10) | 0.226 |
| Total IgE | 129 (12) | 73 (13) | 34 (12) | 105 (15) | 341 (13) | 0.375 |
| Eosinophil count | 272 (25) | 142 (25) | 77 (28) | 186 (26) | 677 (26) | 0.798 |
| 6-min walk test | 104 (10) | 101 (18) | 36 (13) | 176 (25) | 417 (16) | <0.001 |
| Specific rehabilitation program | 27 (3) | 54 (9) | 18 (6) | 135 (19) | 234 (9) | <0.001 |
| BODE index | 77 (7) | 69 (12) | 27 (10) | 134 (19) | 307 (12) | <0.001 |
| Table 2b: Tests Performed by GesEPOC Group | ||||||
|---|---|---|---|---|---|---|
| GesEPOC Phenotype | Total (n=2628) | P-Valuea | ||||
| ACO (n=169) | Non-Exacerbator (n=1639) | Exacerbator with Bronchitis (n=567) | Exacerbator Without Bronchitis (n=253) | |||
| Chest CT | 78 (46) | 785 (48) | 349 (62) | 148 (58) | 1360 (52) | <0.001 |
| Diffusion test for CO | 48 (28) | 438 (27) | 158 (28) | 79 (31) | 723 (28) | 0.501 |
| Lung volumes | 42 (25) | 317 (19) | 129 (23) | 58 (23) | 546 (21) | 0.121 |
| AAT levels | 50 (30) | 372 (23) | 181 (32) | 66 (26) | 669 (25) | <0.001 |
| Respiratory allergy profile | 77 (46) | 123 (8) | 45 (8) | 28 (11) | 273 (10) | <0.001 |
| Total IgE | 71 (42) | 172 (10) | 67 (12) | 35 (14) | 345 (13) | <0.001 |
| Eosinophil count | 60 (36) | 412 (25) | 132 (23) | 70 (28) | 674 (26) | 0.012 |
| 6-min walk test | 24 (14) | 216 (13) | 122 (22) | 55 (22) | 417 (16) | <0.001 |
| Specific rehabilitation program | 16 (9) | 85 (5) | 95 (17) | 41 (16) | 237 (9) | <0.001 |
| BODE index | 20 (12) | 155 (9) | 95 (17) | 37 (15) | 307 (12) | <0.001 |
Data expressed as mean (SD) or n (%).
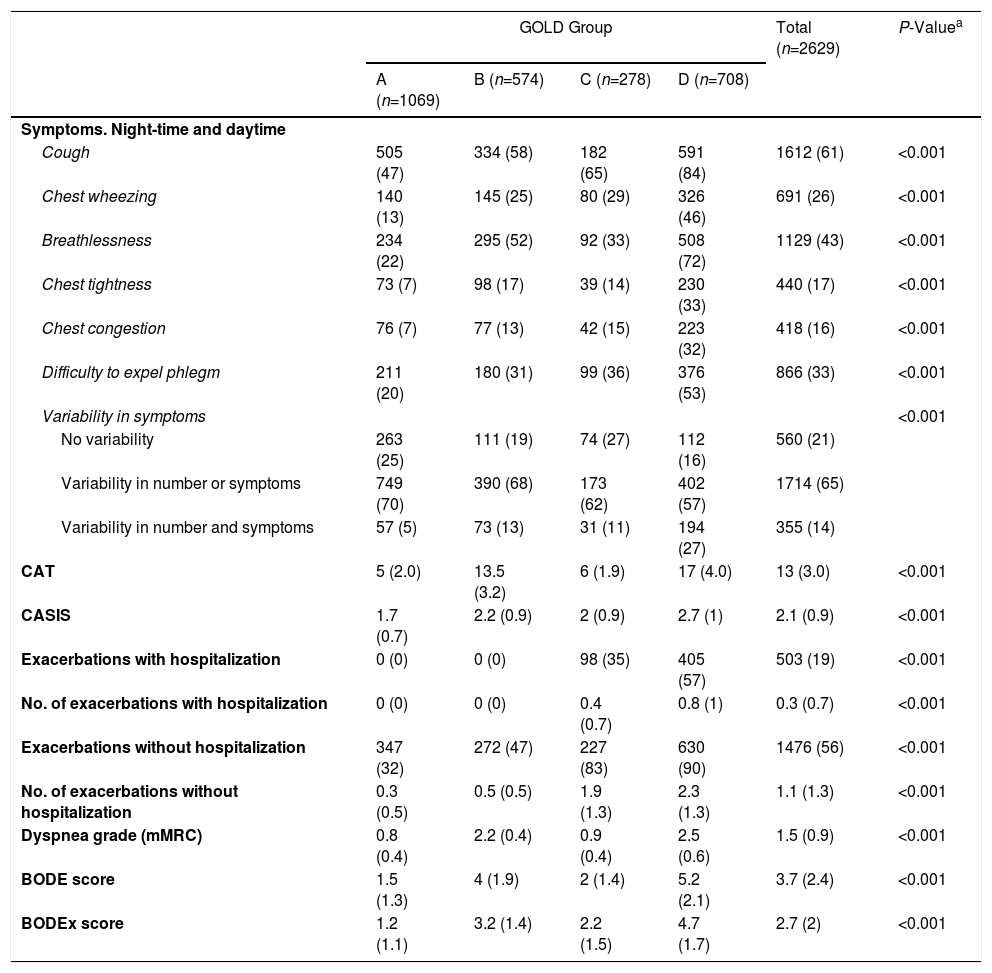
The clinical impact of COPD (symptoms and exacerbations) in the different study populations is analyzed in Table 3. As might be predicted, patients in GOLD groups B and D showed a higher frequency of respiratory symptoms and worse CAT and CASIS scores (Table 3a). With regard to phenotypes, the more symptomatic patients were those with ACO or an exacerbator phenotype with chronic bronchitis. These phenotypes also had significantly worse CAT and CASIS scores (Table 3b). Some differences in exacerbations were also expected, due to the criteria used to classify the groups. The incidence of exacerbations was remarkably low, particularly those that required hospital admission. Of our sample, only 34% had 2 or more exacerbations, and 19% had at least 1 hospitalization.
Exacerbations and Symptoms by GOLD Group.
| GOLD Group | Total (n=2629) | P-Valuea | ||||
|---|---|---|---|---|---|---|
| A (n=1069) | B (n=574) | C (n=278) | D (n=708) | |||
| Symptoms. Night-time and daytime | ||||||
| Cough | 505 (47) | 334 (58) | 182 (65) | 591 (84) | 1612 (61) | <0.001 |
| Chest wheezing | 140 (13) | 145 (25) | 80 (29) | 326 (46) | 691 (26) | <0.001 |
| Breathlessness | 234 (22) | 295 (52) | 92 (33) | 508 (72) | 1129 (43) | <0.001 |
| Chest tightness | 73 (7) | 98 (17) | 39 (14) | 230 (33) | 440 (17) | <0.001 |
| Chest congestion | 76 (7) | 77 (13) | 42 (15) | 223 (32) | 418 (16) | <0.001 |
| Difficulty to expel phlegm | 211 (20) | 180 (31) | 99 (36) | 376 (53) | 866 (33) | <0.001 |
| Variability in symptoms | <0.001 | |||||
| No variability | 263 (25) | 111 (19) | 74 (27) | 112 (16) | 560 (21) | |
| Variability in number or symptoms | 749 (70) | 390 (68) | 173 (62) | 402 (57) | 1714 (65) | |
| Variability in number and symptoms | 57 (5) | 73 (13) | 31 (11) | 194 (27) | 355 (14) | |
| CAT | 5 (2.0) | 13.5 (3.2) | 6 (1.9) | 17 (4.0) | 13 (3.0) | <0.001 |
| CASIS | 1.7 (0.7) | 2.2 (0.9) | 2 (0.9) | 2.7 (1) | 2.1 (0.9) | <0.001 |
| Exacerbations with hospitalization | 0 (0) | 0 (0) | 98 (35) | 405 (57) | 503 (19) | <0.001 |
| No. of exacerbations with hospitalization | 0 (0) | 0 (0) | 0.4 (0.7) | 0.8 (1) | 0.3 (0.7) | <0.001 |
| Exacerbations without hospitalization | 347 (32) | 272 (47) | 227 (83) | 630 (90) | 1476 (56) | <0.001 |
| No. of exacerbations without hospitalization | 0.3 (0.5) | 0.5 (0.5) | 1.9 (1.3) | 2.3 (1.3) | 1.1 (1.3) | <0.001 |
| Dyspnea grade (mMRC) | 0.8 (0.4) | 2.2 (0.4) | 0.9 (0.4) | 2.5 (0.6) | 1.5 (0.9) | <0.001 |
| BODE score | 1.5 (1.3) | 4 (1.9) | 2 (1.4) | 5.2 (2.1) | 3.7 (2.4) | <0.001 |
| BODEx score | 1.2 (1.1) | 3.2 (1.4) | 2.2 (1.5) | 4.7 (1.7) | 2.7 (2) | <0.001 |
| Table 3b: Exacerbations and Symptoms by GesEPOC Group | ||||||
|---|---|---|---|---|---|---|
| GesEPOC Phenotype | Total (n=2628) | P-Valuea | ||||
| ACO (n=169) | Non-Exacerbator (n=1639) | Exacerbator with Bronchitis (n=567) | Exacerbator Without Bronchitis (n=253) | |||
| Symptoms. Night-time and daytime | ||||||
| Cough | 116 (69) | 828 (51) | 499 (88) | 162 (65) | 1605 (61) | <0.001 |
| Chest wheezing | 76 (45) | 264 (16) | 281 (50) | 68 (27) | 689 (26) | <0.001 |
| Breathlessness | 68 (40) | 527 (32) | 385 (68) | 143 (58) | 1123 (43) | <0.001 |
| Chest tightness | 34 (20) | 165 (10) | 193 (34) | 46 (19) | 438 (17) | <0.001 |
| Chest congestion | 13 (8) | 159 (10) | 223 (39) | 23 (9) | 418 (16) | <0.001 |
| Difficulty to expel phlegm | 65 (38) | 378 (23) | 377 (66) | 45 (18) | 865 (33) | <0.001 |
| Variability in symptoms | <0.001 | |||||
| No variability | 34 (20) | 382 (23) | 93 (16) | 53 (21) | 562 (21) | |
| Variability in number or symptoms | 118 (70) | 1126 (69) | 295 (52) | 173 (68) | 1712 (65) | |
| Variability in number and symptoms | 17 (10) | 131 (8) | 179 (32) | 27 (11) | 354 (13) | |
| CAT | 13 (3.1) | 10 (2.2) | 20 (4.3) | 12 (2.1) | 12 (2.0) | <0.001 |
| CASIS | 2.2 (0.9) | 1.9 (0.8) | 2.8 (1) | 2.2 (0.9) | 2.1 (0.9) | <0.001 |
| Exacerbations with hospitalization | 34 (20) | 87 (5) | 265 (47) | 109 (43) | 495 (19) | <0.001 |
| No. of exacerbations with hospitalization | 0.3 (0.5) | 0.1 (0.2) | 0.7 (1) | 0.6 (0.9) | 0.3 (0.7) | <0.001 |
| Exacerbations without hospitalization | 115 (68) | 575 (35) | 554 (98) | 238 (94) | 1482 (56) | <0.001 |
| No. of exacerbations without hospitalization | 1.3 (1.2) | 0.4 (0.5) | 2.6 (1.2) | 2.2 (1.2) | 1.1 (1.3) | <0.001 |
| Dyspnea grade (mMRC) | 1.6 (0.8) | 1.3 (0.8) | 2.2 (0.9) | 1.8 (0.9) | 1.5 (0.9) | <0.001 |
| BODE score | 2.9 (1.7) | 2.8 (2.1) | 5.2 (2.2) | 4.1 (2.3) | 3.7 (2.4) | <0.001 |
| BODEx score | 2.9 (1.7) | 1.9 (1.6) | 4.4 (2.1) | 3.5 (1.9) | 2.7 (2) | <0.001 |
CAT: COPD Assessment Test.
Data expressed as mean (SD) or n (%).
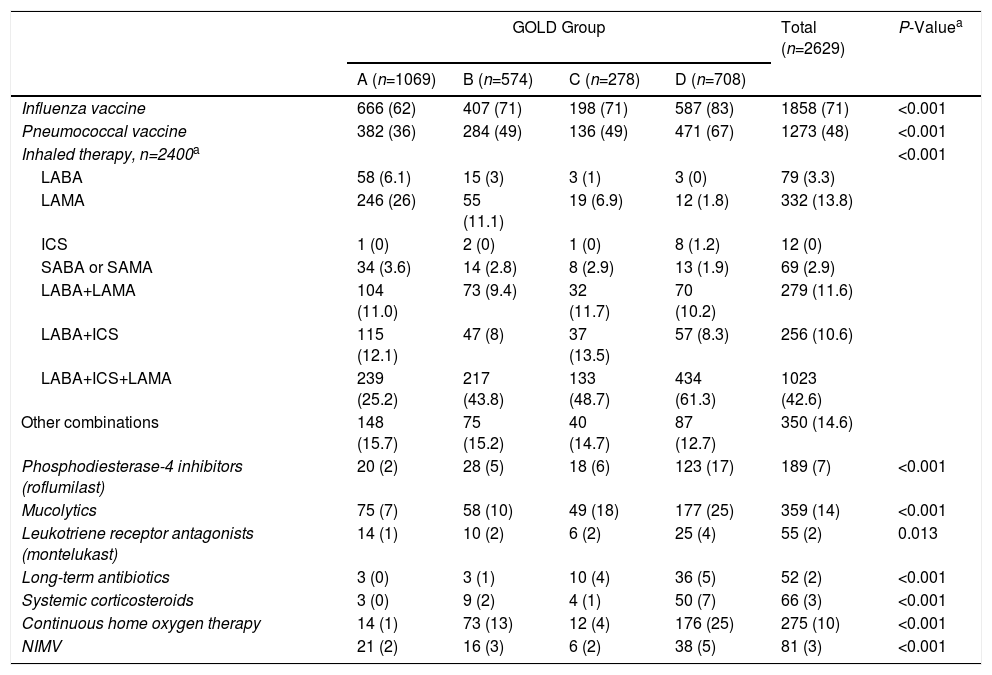
Table 4 shows active COPD treatments at the time of the visit. Rates of influenza virus immunization were high: over 70% of the study population, and more than 80% of higher-risk populations were protected. Treatment was based on different combinations of bronchodilators and inhaled corticosteroids, and more patients received LABA/LAMA in fixed combinations and fewer received inhaled corticosteroids than in previous series. Inhaled corticosteroids were widely used in GOLD groups A and B and in GesEPOC non-exacerbators.
Treatments by GOLD Group.
| GOLD Group | Total (n=2629) | P-Valuea | ||||
|---|---|---|---|---|---|---|
| A (n=1069) | B (n=574) | C (n=278) | D (n=708) | |||
| Influenza vaccine | 666 (62) | 407 (71) | 198 (71) | 587 (83) | 1858 (71) | <0.001 |
| Pneumococcal vaccine | 382 (36) | 284 (49) | 136 (49) | 471 (67) | 1273 (48) | <0.001 |
| Inhaled therapy, n=2400a | <0.001 | |||||
| LABA | 58 (6.1) | 15 (3) | 3 (1) | 3 (0) | 79 (3.3) | |
| LAMA | 246 (26) | 55 (11.1) | 19 (6.9) | 12 (1.8) | 332 (13.8) | |
| ICS | 1 (0) | 2 (0) | 1 (0) | 8 (1.2) | 12 (0) | |
| SABA or SAMA | 34 (3.6) | 14 (2.8) | 8 (2.9) | 13 (1.9) | 69 (2.9) | |
| LABA+LAMA | 104 (11.0) | 73 (9.4) | 32 (11.7) | 70 (10.2) | 279 (11.6) | |
| LABA+ICS | 115 (12.1) | 47 (8) | 37 (13.5) | 57 (8.3) | 256 (10.6) | |
| LABA+ICS+LAMA | 239 (25.2) | 217 (43.8) | 133 (48.7) | 434 (61.3) | 1023 (42.6) | |
| Other combinations | 148 (15.7) | 75 (15.2) | 40 (14.7) | 87 (12.7) | 350 (14.6) | |
| Phosphodiesterase-4 inhibitors (roflumilast) | 20 (2) | 28 (5) | 18 (6) | 123 (17) | 189 (7) | <0.001 |
| Mucolytics | 75 (7) | 58 (10) | 49 (18) | 177 (25) | 359 (14) | <0.001 |
| Leukotriene receptor antagonists (montelukast) | 14 (1) | 10 (2) | 6 (2) | 25 (4) | 55 (2) | 0.013 |
| Long-term antibiotics | 3 (0) | 3 (1) | 10 (4) | 36 (5) | 52 (2) | <0.001 |
| Systemic corticosteroids | 3 (0) | 9 (2) | 4 (1) | 50 (7) | 66 (3) | <0.001 |
| Continuous home oxygen therapy | 14 (1) | 73 (13) | 12 (4) | 176 (25) | 275 (10) | <0.001 |
| NIMV | 21 (2) | 16 (3) | 6 (2) | 38 (5) | 81 (3) | <0.001 |
| Table 4b: Treatment by GesEPOC Group | ||||||
|---|---|---|---|---|---|---|
| GesEPOC Phenotype | Total (n=2628) | P-Valuea | ||||
| ACO (n=169) | Non-Exacerbator (n=1639) | Exacerbator with Bronchitis (n=567) | Exacerbator Without Bronchitis (n=253) | |||
| Influenza vaccine | 132 (78) | 1066 (65) | 469 (83) | 195 (77) | 1862 (71) | <0.001 |
| Pneumococcal vaccine | 90 (53) | 663 (40) | 372 (66) | 148 (58) | 1273 (48) | <0.001 |
| Inhaled therapy, n=2400b | <0.001 | |||||
| LABA | 5 (2.4) | 73 (5.2) | 2 (0) | 1 (0) | 81 (3.3) | |
| LAMA | 4 (2) | 305 (21.8) | 12 (2.2) | 11 (4.5) | 332 (13.8) | |
| ICS | 0 (0) | 4 (0) | 3 (0) | 5 (2.1) | 12 (0) | |
| SABA or SAMA | 2 (0) | 50 (3) | 12 (2.1) | 4 (2) | 68 (2.8) | |
| LABA+LAMA | 11 (5.4) | 190 (13.7) | 61 (11) | 18 (7.4) | 280 (11.7) | |
| LABA+ICS | 46 (22.7) | 132 (13.5) | 46 (8.2) | 34 (14.0) | 258 (10.8) | |
| LABA+ICS+LAMA | 94 (46.5) | 452 (32.3) | 349 (62.8) | 122 (50.4) | 1017 (42.4) | |
| Other combinations | 40 (19.8) | 195 (13.9) | 70 (12.5) | 47 (19.4) | 352 (14.7) | |
| Phosphodiesterase-4 inhibitors (roflumilast) | 8 (5) | 45 (3) | 106 (19) | 30 (12) | 189 (7) | <0.001 |
| Mucolytics | 14 (8) | 136 (8) | 171 (30) | 37 (15) | 358 (14) | <0.001 |
| Leukotriene receptor antagonists (montelukast) | 33 (20) | 13 (1) | 9 (2) | 2 (1) | 57 (2) | <0.001 |
| Long-term antibiotics | 1 (1) | 7 (0) | 35 (6) | 9 (4) | 52 (2) | <0.001 |
| Systemic corticosteroids | 10 (6) | 12 (1) | 31 (5) | 13 (5) | 66 (3) | <0.001 |
| Continuous home oxygen therapy | 14 (8) | 97 (6) | 120 (21) | 42 (17) | 273 (10) | <0.001 |
| NIMV | 8 (5) | 38 (2) | 24 (4) | 13 (5) | 83 (3) | 0.015 |
Data expressed as n (%).
ICS: inhaled corticosteroids; LABA: long-acting β2-agonists; LAMA: long-acting anticholinergics; SABA: short-acting β2-agonists; SAMA: short-acting anticholinergics.
The use of methylxanthines was rare (7%), as was the use of leukotriene receptor antagonists, which was restricted mainly to the ACO phenotype.
DiscussionThis cross-sectional study confirms that the demographic characteristics of COPD patients seen in respiratory clinics in Spain has not changed substantially in the past 10 years.6 Most patients are men aged between 65 and 70 years, who are overweight with a BMI of 27.3, and a mean FEV1 of around 50%. A previous history of smoking was a requirement for inclusion in our study, so our results cannot be compared with those from other series which include patients with chronic airflow obstruction irrespective of their previous history of smoking, or those seen in different care settings.15 However, at the time of the assessment, 22% of our patients were active smokers, and smoking rates were very similar among the more severe GOLD groups and the GesEPOC exacerbators. While still worrying, these data are a significant improvement on those reported in a recent study conducted in different care settings in Spain, which described a prevalence of active smoking of 55% among COPD patients. Despite this discrepancy, our data are consistent with other recent series, in which the prevalence of active smoking ranged around 25% in populations of both primary care and pulmonology patients16 and exclusively pulmonology patients.17
Several cross-sectional observational and case-control studies have reported a high prevalence of concomitant diseases among COPD patients, and the presence of at least 1 associated disease has been described in 88% of cases.18 Regardless of whether there is a causal relationship between COPD and some of these comorbidities, or if they are incidental, some diseases in particular increase both the complexity and the economic burden of the disease, are associated with a higher rate of exacerbations, and represent an important predictor of mortality.19 Although the most common comorbidities were hypertension and dyslipidemia, the high prevalence of associated heart disease (mainly ischemic heart disease and arrhythmias) and anxiety and/or depression, particularly in the more severe GOLD groups and in the GesEPOC exacerbators with chronic bronchitis, must be noted. The impact of heart disease on COPD symptoms and risk is well known,20 but we must not forget that both anxiety and depression, present in almost 30% of patients, can have a very significant effect on the expression of this disease, which moreover is often classified with the use of subjective scales. Both GOLD and GesEPOC base their classifications on the presence of exacerbations; however, despite the association between gastroesophageal reflux and exacerbations detected in our series, there were no differences between the groups.
Although most patients could be stratified and classified according to both GOLD and GesEPOC, the percentage of patients for whom this classification was already available in their records was remarkably low. This is important if we take into account the impact that this may have on treatment.
With regard to phenotype, almost two-thirds of the patients included were non-exacerbators. This proportion is practically identical to that previously reported in other series in Spain11,14,21 and other countries.22 The low percentage of patients in the ACO group (6.4%) is of interest. Although it is generally accepted that this percentage is around 15%,21,23 the lack of well-defined criteria means that unless criteria are specifically predefined, rates in the literature can vary widely.24,25 Non-exacerbators have a lower incidence of symptoms and less variability. COPD is a disease characterized by chronic respiratory symptoms, but previous studies have shown wide variability in the perception of symptoms.8 In our study, we found that the more symptomatic patients (GOLD groups B and D) showed more variability, as did exacerbators with chronic bronchitis in the GesEPOC classification system. Previous authors observed an association between the intensity of symptoms and greater variability, and these studies also found a relationship between variability and more frequent exacerbations.26,27
A very important change in the latest revision of the GesEPOC guidelines involved the introduction of levels of complexity, with different recommendations for the primary care setting and for specialized pulmonology clinics. It seems logical that when a patient is referred to a specialist, it is because he or she requires a more accurate assessment and individualized treatment.28 Characterization of such patients often requires procedures not available in primary care. However, in our study, when patients were grouped by GOLD 2013 and GesEPOC criteria, the percentage with “low-complexity” COPD seen in outpatient pulmonology clinics was high (41% of GOLD group A and 62% of GesEPOC non-exacerbators). It should be noted, however, that in cross-sectional studies such as ours, it is impossible to establish whether the patient was non-complex from the start or if he or she is no longer complex thanks to correct clinical management.
The use of extremely valuable clinical tests in pulmonology clinics, such as diffusion testing or lung volume determinations, is remarkably low, while the use of other tests, such as the 6-minute walk test and the BODE index, on which clinical guidelines base their intervention strategies, is marginal (16% and 15%, respectively). The reason for the underuse of these techniques must be analyzed, and it must be determined if the new GesEPOC stratification will reverse this situation or if it is simply a case of specialists failing to perceive the clinical value of these examinations. In the same vein, it is equally surprising that AAT levels were determined in only 25% of patients. These results are consistent with the low frequency of AAT testing in population studies,29 despite the fact that existing guidelines recommend that it is analyzed at least once in all COPD patients.7,30
Both GesEPOC and GOLD recommend treatment escalation, adapted to the characteristics of the patient.7,30 For many years, the treatment of COPD was limited to symptomatic measures. The need to reduce risk, particularly of exacerbations, prompted the introduction of inhaled corticosteroids and other anti-inflammatory drugs. This joint approach, addressing both symptoms and risk, popularized the combined use of bronchodilators and inhaled corticosteroids, and for many years this was the core treatment in the large majority of COPD patients.31 However, recent data have placed emphasis on the role of bronchodilators, particularly long-acting anticholinergics, in reducing exacerbations.32 This, coupled with the need to optimize the use of inhaled corticosteroids with the prescription of low doses in carefully selected patients who are most likely to benefit from these or other more marginal drugs, has led to support for the optimization of bronchodilation and individualized treatment guided by patient profiles.33
Our study data reflected this changing therapeutic pattern compared to previous studies performed in primary care in Spain34 and neighboring countries, such as the United Kingdom,35 although the use of inhaled corticosteroids remains widespread (53%). It should be noted that, in accordance with the recommendations, practically 100% of ACO patients are treated with inhaled corticosteroids. Moreover, while escalation treatment exists, inasmuch as more serious patients receive more treatment, over 30% of GOLD group A patients continue to receive inhaled corticosteroids. Up to 25% receive treatment with dual bronchodilation, although there is now general consensus on the benefit of this strategy.
A positive finding of this study relates to the implementation of the preventive measures set out in the COPD guidelines, which specify the need for a universal vaccination plan in all patients with COPD. Rates of influenza virus immunization in our series were high: over 70% of the study population, and more than 80% of higher risk populations were protected. The opposite is true of other recommendations such as specific rehabilitation programs, which are still rarely implemented, even in seriously ill patients.
The main limitation of our study is its cross-sectional design and retrospective analysis of some variables. This might have been undermined the quality of some data (number and type of exacerbations), but no detailed analyses were presented in such cases. Another limitation is the potential variability in the classification of patients, especially with the GOLD criteria. Shortly after the GOLD A-D classification scheme was published, multiple studies emerged demonstrating the limitations of this method and the inconsistencies that arose when different criteria were used to evaluate symptoms. The same occurred with lung function and exacerbations in group D. The objective of our study was not to address this problem, so classifications were made using the GOLD criteria nonetheless, as was recommended at the time of the study. Finally, it should be remembered that the selected population consisted exclusively of patients seen in pulmonology consultations, so the results cannot be necessarily extrapolated to COPD patients seen in other healthcare settings.
ConclusionThe results of this study conducted exclusively in respiratory clinics show that a high proportion of patients had not been previously stratified as recommended by both the GOLD and GesEPOC guidelines, even though most could be classified in the context of the study. The proportion of non-complex patients seen in these clinics was also found to be very high, and the use of respiratory tests, other than forced spirometry, was very low. Therapeutic regimens have changed slightly compared to previous series, and comorbidities that might modify patients’ clinical presentation were common. These findings can help identify areas for improvement and will serve as a starting point for determining the impact of clinical guidelines on the management of COPD after publication.
Conflict of InterestsJosé Luis Izquierdo has received honoraria for speaking engagements and consultancy fees from AstraZeneca, Bayer, Boehringer-Ingelheim, Chiesi, Glaxo Smith Kline, Menarini, Novartis, Pfizer, Sandoz and Teva.
Marc Miravitlles has received honoraria for speaking engagements from Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, CSL Behring, Grifols and Novartis; scientific consultancy fees from Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, CSL Behring, Laboratorios Esteve, Mereo Biopharma, Verona Pharma, pH Pharma, Novartis and Grifols, and research funding from GlaxoSmithKline and Grifols.
Cristina Esquinas has received honoraria for consultancy activities and scientific collaborations from Bayer and speaking fees from CSL Behring.
Maite Pérez is an employee of AstraZeneca.
Myriam Calle has received honoraria for speaking engagements from Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, Menarini, and Novartis, and for scientific consultancy activities from GlaxoSmithKline, Chiesi and Novartis.
José Luis López-Campos has received honoraria for speaking engagements, scientific consultancy, participation in clinical studies, and preparation of publications from (in alphabetical order): Almirall, AstraZeneca, Bayer, Boehringer Ingelheim, Cantabria Pharma, Chiesi, Esteve, Faes, Ferrer, Gebro, GlaxoSmithKline, Grifols, Menarini, MSD, Novartis, Pfizer, Rovi, Teva and Takeda.
José Miguel Rodríguez González-Moro has received honoraria for consultancy activities and scientific collaborations from Boehringer Ingelheim, GSK, Rovi, Menarini, Orión, Teva, Ferrer and Grifols.
Ciro Casanova in the last 3 years, has received honoraria for speaking engagements, scientific consultancy, participation in clinical studies, and preparation of publications from (in alphabetical order): AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Gebro, GlaxoSmithKline, Menarini, Novartis, Rovi and Teva.
Cristóbal Esteban has received honoraria for consultancy activities and scientific collaborations from AstraZeneca, Boehringer Ingelheim, Chiesi, Gebro, and GlaxoSmithKline.
Pilar de Lucas has no conflict of interest.
Please cite this article as: Izquierdo JL, Miravitlles M, Esquinas C, Pérez M, Calle M, López Campos JL, et al. Características de los pacientes con EPOC tratados en neumología en España según grupos GOLD y fenotipos clínicos GesEPOC. Arch Bronconeumol. 2018;54:559–567.