Community-acquired pneumonia (CAP) continues to represent a significant burden on global public health, with substantial morbidity and mortality.1,2 Despite advancements in diagnostics and therapeutics, uncertainties persist regarding the optimal duration of antibiotic therapy, particularly for hospitalized patients.
Antibiotic stewardship is a cornerstone in the management of infectious diseases, aiming to optimize patient outcomes by improving adherence and minimizing the risks associated with antibiotic overuse, such as the emergence of antimicrobial resistance and adverse drug reactions.3 In recent years, clinical trials and meta-analyses have suggested that shorter courses of antibiotics may be as effective as longer durations in achieving clinical cure for patients with CAP.4–6 These findings have been reflected in guideline recommendations.7
Despite this, physicians struggle with reducing treatment durations, as shown in some studies in which up to 70–93% of patients are prescribed excessive treatment durations.8,9 Evidence to evaluate short regimens in a real-life setting could enhance practitioner's confidence in short treatments, which is why we designed this study.
The objectives were to compare 30-day mortality and 30-day readmissions between patients receiving a short-course treatment (7 days) and patients receiving longer courses (8–10 days) for CAP that require hospitalization.
We conducted a retrospective cohort study. Briefly, we included consecutive adult patients hospitalized during two one-year periods: 2007–2008 and 2017–2018. These patients belonged to the cohort of a previously published study.10 Basal characteristics, etiology and outcomes remained consistent between the two periods. Subjects with aspiration or nosocomial pneumonia were excluded. Those who received treatment for less than 7 days were also excluded to prevent selection bias, as their treatment duration was mostly not influenced by physician‘s decision, often resulting from transfers to other facilities or death. Participants presenting complicated CAP (empyema, necrotizing pneumonia, or abscess) were not included as they should receive longer treatment regimens. Patients were categorized based on treatment duration: 7-day (short-course) or 8–10-day (long-course).
We performed an unvaried and a multivariate analysis (with Lasso methodology for variable selection) by binary logistic regression to identify variables independently associated with mortality and readmission. Non-redundant variables with a p value≤0.05 on the unvaried analysis, or those considered clinically relevant, were included in the multivariate analysis. A propensity score analysis by Inverse Probability of Treatment Weighting (IPTW) was used to match both groups and to assess an adjusted mortality and readmission analysis. Variables included in the propensity-score model were septic shock, PSI mortality class, age, radiological pattern (bilateral vs unilateral consolidation), respiratory failure, ICU admission, any comorbidity, CURB-65, bacteremia, bacterial etiology, Staphylococcus aureus etiology, any immunosuppressive condition, nursing home, flu-season (November–March, both included), flu-vaccine, smoking, Streptococcus pneumoniae vaccine, sex and alcohol consumption. Statistical analyses were performed using the IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY; IBM Corp. Released 2011 and Stata 15.1.
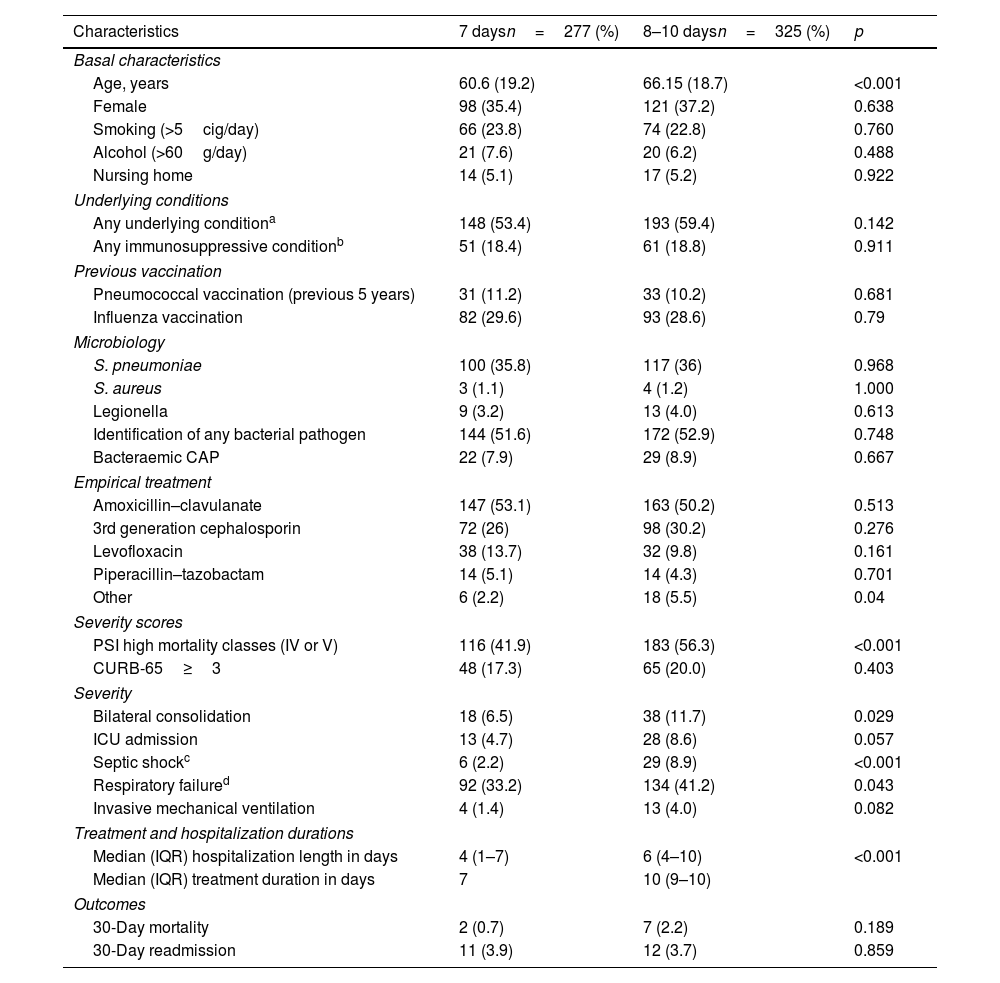
Overall, 602 patients were enrolled, 277 in the short-course group and 325 in the prolonged-course group. The mean age was 63.6 years (standard deviation 19.1) and 220 (36.4%) were women. Table 1 shows baseline characteristics and clinical parameters of patients receiving short vs long treatment courses. There were no differences in terms of sex, underlying conditions and immunosuppressive factors between both groups, but patients receiving longer treatments were slightly older (60.6 vs 66.2 years, p<0.001). In terms of severity, patients with bilateral involvement (p=0.029), septic shock (p<0.001), respiratory failure (p=0.037) or those who presented higher PSI scores (p<0.001) received more frequently long antibiotic courses.
Baseline characteristics, outcomes and other relevant factors of patients that received short and long antibiotic courses.
| Characteristics | 7 daysn=277 (%) | 8–10 daysn=325 (%) | p |
|---|---|---|---|
| Basal characteristics | |||
| Age, years | 60.6 (19.2) | 66.15 (18.7) | <0.001 |
| Female | 98 (35.4) | 121 (37.2) | 0.638 |
| Smoking (>5cig/day) | 66 (23.8) | 74 (22.8) | 0.760 |
| Alcohol (>60g/day) | 21 (7.6) | 20 (6.2) | 0.488 |
| Nursing home | 14 (5.1) | 17 (5.2) | 0.922 |
| Underlying conditions | |||
| Any underlying conditiona | 148 (53.4) | 193 (59.4) | 0.142 |
| Any immunosuppressive conditionb | 51 (18.4) | 61 (18.8) | 0.911 |
| Previous vaccination | |||
| Pneumococcal vaccination (previous 5 years) | 31 (11.2) | 33 (10.2) | 0.681 |
| Influenza vaccination | 82 (29.6) | 93 (28.6) | 0.79 |
| Microbiology | |||
| S. pneumoniae | 100 (35.8) | 117 (36) | 0.968 |
| S. aureus | 3 (1.1) | 4 (1.2) | 1.000 |
| Legionella | 9 (3.2) | 13 (4.0) | 0.613 |
| Identification of any bacterial pathogen | 144 (51.6) | 172 (52.9) | 0.748 |
| Bacteraemic CAP | 22 (7.9) | 29 (8.9) | 0.667 |
| Empirical treatment | |||
| Amoxicillin–clavulanate | 147 (53.1) | 163 (50.2) | 0.513 |
| 3rd generation cephalosporin | 72 (26) | 98 (30.2) | 0.276 |
| Levofloxacin | 38 (13.7) | 32 (9.8) | 0.161 |
| Piperacillin–tazobactam | 14 (5.1) | 14 (4.3) | 0.701 |
| Other | 6 (2.2) | 18 (5.5) | 0.04 |
| Severity scores | |||
| PSI high mortality classes (IV or V) | 116 (41.9) | 183 (56.3) | <0.001 |
| CURB-65≥3 | 48 (17.3) | 65 (20.0) | 0.403 |
| Severity | |||
| Bilateral consolidation | 18 (6.5) | 38 (11.7) | 0.029 |
| ICU admission | 13 (4.7) | 28 (8.6) | 0.057 |
| Septic shockc | 6 (2.2) | 29 (8.9) | <0.001 |
| Respiratory failured | 92 (33.2) | 134 (41.2) | 0.043 |
| Invasive mechanical ventilation | 4 (1.4) | 13 (4.0) | 0.082 |
| Treatment and hospitalization durations | |||
| Median (IQR) hospitalization length in days | 4 (1–7) | 6 (4–10) | <0.001 |
| Median (IQR) treatment duration in days | 7 | 10 (9–10) | |
| Outcomes | |||
| 30-Day mortality | 2 (0.7) | 7 (2.2) | 0.189 |
| 30-Day readmission | 11 (3.9) | 12 (3.7) | 0.859 |
Any underlying condition: chronic heart diseases, chronic lung disease, chronic renal disease, chronic neurological disease, cirrhosis, any malignancy with active treatment or HIV with CD4 count<200.
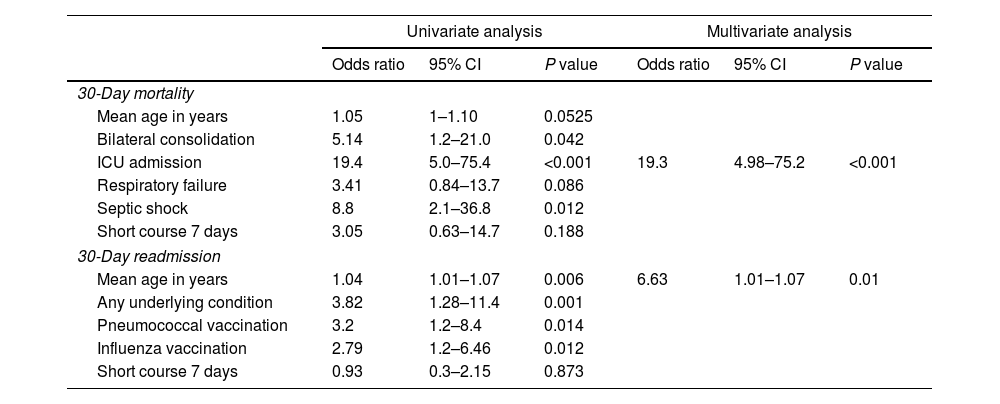
Regarding outcomes, there were no significant disparities in 30-day mortality (p=0.189) or 30-day readmissions (p=0.86) between the short-course and prolonged-course groups. Multivariate analyses revealed that ICU admission was the sole factor associated with 30-day mortality. There was no association between short-course antibiotic treatment and mortality (Table 2). Furthermore, age emerged as the only variable associated with 30-day readmission.
Unadjusted univariate and multivariate analysis of potential risk factors associated with 30-day mortality and 30-day readmission.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| 30-Day mortality | ||||||
| Mean age in years | 1.05 | 1–1.10 | 0.0525 | |||
| Bilateral consolidation | 5.14 | 1.2–21.0 | 0.042 | |||
| ICU admission | 19.4 | 5.0–75.4 | <0.001 | 19.3 | 4.98–75.2 | <0.001 |
| Respiratory failure | 3.41 | 0.84–13.7 | 0.086 | |||
| Septic shock | 8.8 | 2.1–36.8 | 0.012 | |||
| Short course 7 days | 3.05 | 0.63–14.7 | 0.188 | |||
| 30-Day readmission | ||||||
| Mean age in years | 1.04 | 1.01–1.07 | 0.006 | 6.63 | 1.01–1.07 | 0.01 |
| Any underlying condition | 3.82 | 1.28–11.4 | 0.001 | |||
| Pneumococcal vaccination | 3.2 | 1.2–8.4 | 0.014 | |||
| Influenza vaccination | 2.79 | 1.2–6.46 | 0.012 | |||
| Short course 7 days | 0.93 | 0.3–2.15 | 0.873 | |||
A propensity score model was performed to adjust the basal characteristics of both groups and it also failed to uncover significant differences in 30-day mortality (OR:2.23; 95%CI, 0.35–10.9, p=0.325) or readmission (OR:0.89; 95%CI, 0.38–2.09, p=0.79) between patients receiving 7-day versus 8–10-day treatments.
Our study examines a large cohort of adult patients with severe pneumonia, applying a propensity-score model. Results indicate that two robust endpoints, such as 30-day mortality and 30-day readmissions are similar in subjects treated for 7 days and those treated 8–10 days. It is noteworthy that, among the patients who received a short treatment-course, 40% presented a PSI score class IV or V and even 4.7% of them were admitted in the ICU.
Different clinical trials have addressed the issue of treatment duration in adult patients with CAP. Trials comparing 5 or 3 days with longer courses yielded similar clinical success rates but enrolled predominantly low risk patients.5,11 A Spanish study that randomized patients to stop or continue with antibiotics after 5 days presented similar results, however most of the patients received quinolone-based treatments, and the findings should not be extrapolated to β-lactam-based regimens.6 Dinh et al. published a clinical trial in which a 3-day course with β-lactams was compared to longer treatments and found non-inferiority of short treatments. Interestingly, this study included a high proportion of subjects with PSI score classes IV or V, however more than 75% did not present any comorbid condition.4
Although clinical trials offer the highest degree of scientific evidence, they can present problems of external validity since they usually have restrictive selection criteria.12 Several meta-analyses and literature reviews have as well addressed the issue and have found no differences between patients receiving long and short treatment courses.13–15 The main limitation of these analyses is that they include heterogeneous studies, with highly variable treatment regimens, different inclusion criteria and disparate populations.
Observational studies may be more representative of real clinical practice as they include patients that are often excluded in the previously mentioned studies. Despite this, scarce studies focus on real-life experience to investigate the optimal treatment duration in adult patients hospitalized with CAP.16 These studies are prone to biases, such as the confounding by severity bias, in which patients with severe conditions are more likely to receive intensive or longer treatments than other subjects.12 To mitigate this, statistical methods, such as propensity-score models should be applied. The only adjusted study assessing the subject is a recently published Danish study that found that hospitalized patients with CAP and early clinical response presented similar outcomes when they received short versus long-course treatments.17
As an observational retrospective, single center design the present study has limitations. Even though the propensity score model potentially corrects biases, we cannot assure that all factors were adjusted. This is why, we consider that this study does not allow to assure that all patients with severe presentations can be safely treated with a 7-day course. Probably the best strategy to guide duration of therapy depends on the clinical situation of the patients once they have received at least 5–7 days of treatment. Our study contributes insights to the ongoing discourse surrounding the optimal duration of antibiotic therapy for CAP. By endorsing shorter treatment durations in a pragmatic approach, we hope to catalyze practice changes and improve the quality of care for CAP patients.
Conflict of interestThe authors declare no conflicts of interest direct or indirectly related to this work.