Pulmonary thromboendarterectomy is the treatment of choice in chronic thromboembolic pulmonary hypertension. We report our experience with this technique.
MethodsBetween February 1996 and June 2014, we performed 106 pulmonary thromboendarterectomies. Patient population, morbidity and mortality and the long-term results of this technique (survival, functional improvement and resolution of pulmonary hypertension) are described.
ResultsSubjects’ mean age was 53±14 years. A total of 89% were WHO functional class III–IV, presurgery mean pulmonary pressure was 49±13mmHg and mean pulmonary vascular resistance was 831±364dynesscm−5. In-hospital mortality was 6.6%. The most important post-operative morbidity was reperfusion pulmonary injury, in 20% of patients; this was an independent risk factor (P=.015) for hospital mortality. With a 31-month median follow-up (interquartile range: 50), 3- and 5-year survival was 90% and 84% respectively. At 1 year, 91% were WHO functional class I–II; mean pulmonary pressure (27±11mmHg) and pulmonary vascular resistance (275±218dynesscm−5) were significantly lower (P<.05) than before the intervention. Although residual pulmonary hypertension was detected in 14 patients, their survival at 3 and 5 years was 91% and 73%, respectively.
ConclusionsPulmonary thromboendarterectomy offers excellent results in chronic thromboembolic pulmonary hypertension. Long-term survival is good, functional capacity improves, and pulmonary hypertension is resolved in most patients.
La tromboendarterectomía pulmonar es el tratamiento de elección en la hipertensión pulmonar tromboembólica crónica. Presentamos nuestra serie completa con esta técnica.
MétodosDesde febrero de 1996 hasta junio de 2014, hemos realizado 106 tromboendarterectomías. Analizamos las características de la población, la mortalidad y morbilidad asociadas a la técnica y los resultados a largo plazo de supervivencia, mejoría funcional y resolución de la hipertensión pulmonar.
ResultadosLa edad media de la población fue 53±14 años. El 89% estaba en clase funcional III–IV de la OMS. La presión pulmonar media prequirúrgica fue 49±13mmHg y las resistencias vasculares pulmonares 831±364dinasscm−5. La mortalidad hospitalaria fue 6,6%. La morbilidad postoperatoria más relevante fue debida al edema pulmonar por reperfusión en el 20%, que fue factor de riesgo independiente (p=0,015) para mortalidad hospitalaria. Con una mediana de seguimiento de 31 meses (rango intercuartil 50), la supervivencia a los 3 y 5 años es 90 y 84%. Al año de seguimiento, el 91% está en clase funcional I–II de la OMS, la presión pulmonar media en 27±11mmHg y las resistencias pulmonares vasculares en 275±218dinasscm−5 (significativamente menores (p<0,05) que las basales). En 14 pacientes se diagnosticó hipertensión pulmonar persistente; aun así, su supervivencia es, a los 3 y 5 años, 91 y 73%, respectivamente.
ConclusionesLa tromboendarterectomía pulmonar ofrece resultados excelentes en el tratamiento de la hipertensión pulmonar tromboembólica crónica. Proporciona una elevada supervivencia a largo plazo, mejora la capacidad funcional y resuelve la hipertensión pulmonar en la mayoría de los pacientes.
Chronic thromboembolic pulmonary hypertension (CTEPH) is a rare entity, found in just 0.5%–9% of patients with pulmonary embolism (PE).1,2 Various mechanisms behind the development of CTEPH have been suggested: recurrent embolism,3 growth of the thrombus in the bronchial tree4 and vascular disease secondary to endothelial injury.5 Valvular disease, involving microvascular changes that are indistinguishable from idiopathic pulmonary hypertension (PH), can occur in areas of the vascular tree unaffected by PE. The treatment of choice for this disease, wherever possible, consists of pulmonary thromboendarterectomy (PTE) to remove obstructive material from the pulmonary arteries.6–8
The intervention pursues three main goals: hemodynamic stability, by reducing the effect of PH on the right ventricle; respiratory stability, improving ventilatory efficiency by relieving alveolar dead space; and prophylaxis, by preventing right ventricle failure, retrograde propagation of thrombi in the bronchial tree, and secondary valvular disease in permeable vessels.
We present the results of the first 106 PTEs performed in the Hospital Universitario 12 de Octubre. We report the immediate postoperative results in terms of mortality, morbidity and hemodynamic parameters. We also analyze results of long-term follow-up in terms of survival, functional capacity, hemodynamic parameters and right ventricle remodeling.
Patients and MethodsPopulationIndications for surgery were9,10: (a) CTEPH at WHO functional class III–IV; (b) pulmonary vascular resistance (PVR) higher than 300dynasscm−5 or below this level but with documented exercise PH (c) surgically accessible thrombi or characteristic lesions (webs, bands, intimal irregularities) in the main bronchial, lobular and proximal segmental branches. The correlation between PVR levels, perfusion defects in the ventilation/perfusion lung scan, and pulmonary angiography findings was considered. As a requirement for surgery, all patients had to have received anticoagulation therapy for at least 3 months prior to the intervention. The decision to operate was always taken in a meeting of members of the Multidisciplinary Pulmonary Hypertension Team.
Exclusion criteria were no surgical access and serious comorbidity (severe pulmonary disease, malignant neoplasm, etc.)
Implantation of an inferior vena cava filter was evaluated on a case-by-case basis.
DiagnosisThe initial diagnostic protocol was the same as that used in other PH etiologies, with ventilation/perfusion lung scan as the screening technique for establishing the etiology of the CTEPH.10–12 Pulmonary angiography with selective catheterization of lobular branches was the gold standard for diagnosis and confirmation of CTEPH13–15; right heart catheterization was performed at the same time. All patients also underwent contrast-enhanced multislice computed tomography.16,17
Patients aged over 45 years or with risk factors for coronary artery disease underwent coronary angiography.
Functional status was assessed objectively with the 6-min walk test (6MWT) and ergospirometry with oxygen uptake. All patients were also tested for thrombophilia.
Surgical TechniquePTE was performed in accordance with the University of California (San Diego, USA) protocol.5,18 The two guiding principles of the intervention were that it must involve a bilateral procedure, and consist of a full endarterectomy, not merely an embolectomy.
Surgical approach was through median sternotomy, and the PTE was performed with full cardiopulmonary bypass, aortic cross-clamping and deep, 20°C, hypothermia. Endarterectomy was performed during 10-min periods of circulatory arrest, followed by reperfusion lasting at least 5min. A Hopkins II (Karl Storz, Tuttlingen, Germany) angioscope connected to a Twinvideo (Karl Storz, Tuttlingen, Germany) was used to optimize visibility and illuminate the surgical field.
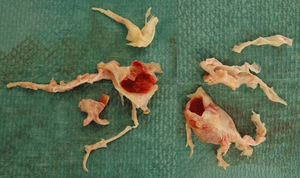
The biological material extracted during the PTE (Fig. 1) was grouped according to histopathological prognostic value established by the University of California group (San Diego, USA)19: type 1, fresh thrombus in the main-lobar pulmonary arteries; type 2, intimal thickening and fibrosis proximal to the segmental arteries, with no thrombus; type 3, disease within distal segmental arteries only; and type 4, distal arteriolar vascular disease.
Postoperative PeriodWe defined lung reperfusion injury as postoperative respiratory failure causing hypoxia and accompanied by pulmonary infiltrates on chest X-ray in some of the surgical areas, needing mechanical ventilation lasting more than 96h.
Follow-upThe follow-up protocol was first check-up at 1 month, with physical examination, blood work and 6MWT; second check-up at 6 months, with physical examination, blood work, echocardiogram, ergospirometry with oxygen uptake, and right heart catheterization. Following this, patients attended an annual check-up with physical examination, echocardiogram and 6MWT. We defined diagnosis of residual or persistent post-PTE pulmonary hypertension as a PVP higher than 400dynasscm−5 in the 6-month hemodynamic study. Patients meeting this criterion underwent contrast-enhanced multislice chest computed tomography.
All patients remained on long-term anticoagulant therapy.
StatisticsContinuous variables are shown as mean±standard deviation, or median and interquartile range (IQR), and categorical variables are shown as frequencies. Comparison between categorical variables was analyzed with Pearson's χ2 or Fisher's exact test. Quantitative variables were analyzed using the Student's t or Mann–Whitney U tests, depending on the results of Shapiro–Wilk normality testing. The impact of the following variables on in-hospital mortality was analyzed: specific bridging treatment, functional class IV, PVR>1000dynasscm−5 in preoperative catheterization, development of reperfusion edema, and being among the first 30 patients in this series to undergo surgery. All variables statistically correlated with mortality were analyzed using logistic regression, and univariate and multivariate analyses to calculate their relative risk and 95% confidence interval (95% CI).
Survival curves were calculated using the Kaplan–Meier method and compared using the log rank test.
Statistical significance was set at P<.05.
ResultsWe performed 106 consecutive PTEs between February 1996 and June 2014 in our hospital. The mean age of the population was 53±14 years (range 23–77); 57% were men. The clinical and hemodynamic characteristics of the patients are shown in Table 1. In 29 patients, PVR was higher than 1000dynasscm−5. Seven patients (6.6%) had undergone previous heart surgery; 3 of these interventions were PTE (one of which was performed in our hospital). Mean time between diagnosis of CTEPH and surgery was 5 months (IQR 8).
Preoperative Characteristics of the Sample.
| Variable | Population (n=106) |
|---|---|
| History of PE | 83 (78%) |
| History of DVP | 41 (39%) |
| Hypercoagulability | 55 (52%) |
| WHO functional status | |
| II | 12 (11%) |
| III | 80 (76%) |
| IV | 14 (13%) |
| Stroke | 20 (19%) |
| Hemoptysis | 13 (12%) |
| Six-minute walk test, meters | 384±111 |
| NT-proBNP | 1199±1248 |
| Heart failure | 27 (25%) |
| Patients with specific bridging therapy | 63 (59%) |
| Time from diagnosis to surgery, months | 12±21 |
| Echocardiogram | |
| RV diameter, mm | 44±10 |
| TAPSE | 17±4 |
| Pericardial effusion | 11 (10%) |
| Hemodynamics | |
| RVP, mmHg | 10±6 |
| mPAP, mmHg | 49±13 |
| PVR, dynasscm−5 | 831±364 |
| CO, l/min | 4.2±1 |
CO: cardiac output; DVP: deep vein thrombosis; mPAP: mean pulmonary artery pressure; PE: pulmonary embolism; RV: right ventricle; RVP: right ventricular pressure; PVR: pulmonary vascular resistance; TAPSE: tricuspid annular plane systolic excursion.
Ninety-six (91%) of the PTEs were bilateral. Mean time with cardiopulmonary bypass, ischemia and circulatory arrest was 205±38, 116±28 and 40±14min, respectively. In 20 patients, PTE was combined with another procedure: 7 tricuspid repairs, 7 myocardial revascularizations, 5 permeable oval foramen closures, and 1 right ventricle thrombectomy. Of the biological material extracted, 49% was classified (under the San Diego system) as type 1, 42% as type 2, and 9% as type 3.
Median time on ventilation in the recovery room was 27h (IQR 92). The most relevant morbidity was lung reperfusion injury in 21 patients (20%), pulmonary hemorrhage in 4 patients (4%), reintervention due to bleeding in 6 patients (6%), heart failure in 3 patients (3%), transient ischemic attack in 1 patient (1%), and need for ECMO or ventricular assist device in 5 patients (5%). Indication for ECMO was heart failure in 2 patients, and respiratory failure due to pulmonary hemorrhage in 3 patients. Mean ICU stay was 5 days (IQR 7), and mean hospital stay was 13 days (IQR 9). In the recovery room, mean PAP was 28±7mmHg and mean PVR was 311±130dynasscm−5, significantly (P<.05) lower than preoperative levels.
Overall in-hospital mortality for the series was 6.6% (7/106, 95% CI: 2.8%–13.5%). Causes of mortality were respiratory failure in 4 patients, pulmonary hemorrhage in 2 patients, and cardiogenic shock in 1 patient.
In the univariate analysis, being among the first 30 cases (P=.019), PVR≥1000dynasscm−5 (P=.019), functional class IV (P=.006) and postoperative lung reperfusion injury (P=.003) were associated with in-hospital mortality. Following multivariate analysis, only reperfusion lung injury remained as an independent risk factor for in-hospital mortality (P=.015), (Table 2).
Risk Factors for In-hospital Mortality.
| Risk factor | In-hospital mortality (%) | Univariate analysisRR (95% CI) | Multivariate analysisRR (95% CI) | ||
|---|---|---|---|---|---|
| Factor present | |||||
| Yes | No | P | |||
| First 30 cases | 17 | 3 | .019 | 7.4 (1.3–41) | |
| PVR>1000dynasscm−5 | 17 | 3 | .019 | 7.4 (1.3–41) | |
| WHO functional class IV | 29 | 3 | .006 | 12 (2.3–61) | |
| Reperfusion injury | 24 | 2 | .003 | 13 (2.3–73) | 17 (1.7–171) |
CI: confidence interval; PVR: pulmonary vascular resistance; RR: relative risk.
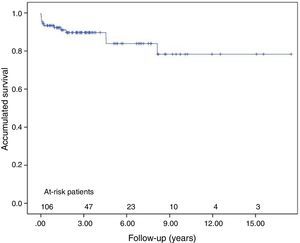
Mean follow-up was 31 months (IQR 50). Survival at 3 and 5 years was 90%±3% and 84%±5% respectively, including in-hospital mortality (Fig. 2). Changes in clinical, echocardiographic and hemodynamic parameters at 1-year follow-up are shown in Table 3.
Clinical, Echocardiographic and Hemodynamic Changes Over Preoperative Baseline at 1 Year of Follow-up.
| Variable | Preoperative | Postoperative | P |
|---|---|---|---|
| WHO functional class | |||
| I–II | 9 (11%) | 78 (91%) | <.001 |
| III–IV | 76 (89%) | 7 (8%) | |
| Six-minute walk test, meters | 382±117 | 469±87 | <.001 |
| NT-pro-BNP | 1266±1369 | 253±280 | <.001 |
| Patients with specific preoperative therapy | 53 (62%) | 18 (21%) | <.001 |
| Echocardiogram | |||
| RV diameter, mm | 45±9 | 32±6 | <.001 |
| Hemodynamics | |||
| RVP, mmHg | 9±6 | 7±4 | .011 |
| sPAP, mmHg | 84±22 | 43±19 | <.001 |
| dPAP, mmHg | 28±8 | 17±8 | <.001 |
| mPAP, mmHg | 48±12 | 27±11 | <.001 |
| PVR, dynasscm−5 | 789±345 | 275±218 | <.001 |
| CO, l/min | 4.3±1 | 5.3±1.4 | <.001 |
CI: confidence interval; CO: cardiac output; dPAP: diastolic pulmonary artery pressure; mPAP: mean pulmonary artery pressure; PVR: pulmonary venous pressure; RVP: right ventricular pressure; sPAP: systolic pulmonary artery pressure; RV: right ventricle.
In total, 6 patients died during follow-up. Causes of death were heart failure (3 patients), infection (1 patient), adenocarcinoma of the bladder (1 patient) and pulmonary thromboembolism (1 patient).
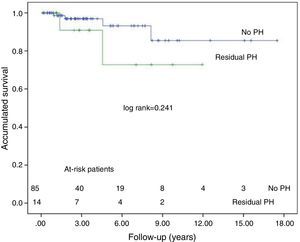
Fourteen patients were diagnosed with persistent PH following PTE. At 1 year, 64% of these patients were classified as functional class I–II. Nine (64%) patients required specific treatment for PH: endothelin receptor antagonists, phosphodiesterase type 5 inhibitors, prostacyclin analogs or soluble guanylate cyclase stimulators. Two patients died due to heart failure. Actuarial survival in the persistent PH group is 91±9 at 3 years, and 73±11 at 5 years. Fig. 3 shows the survival curve for this patient group and that of patients with no residual PH, after adjusting for in-hospital mortality. There is no significant difference between groups. It is interesting to note that despite persistence of PH, PVR was significantly reduced compared to preoperative levels (924±245 vs, 641±269dynasscm−5; P=.033).
DiscussionCTEPH is a rare disease, with only 8.9 cases per million inhabitants in our region.20 Studies have shown that CTEPH is a long-term complication of symptomatic PTE, with an accumulative incidence of 0.1%–9.1% at 2 years.1,2 However, in a significant number of cases, PTE was asymptomatic.5 This is why CTEPH is thought to be under-diagnosed, and patients are all too often referred late for treatment. Clinical guidelines10 and consensus documents15 establish the need to form multidisciplinary teams of expert surgeons to treat CTEPH. Surgery should not be definitively ruled out in any patient until they have been assessed by this team. In the international register,21 up to 43% of the patients evaluated were not considered candidates for surgery, and the same was true of 70% of patients in the Spanish register (REHAP).20 Our team has performed surgery on 53%22 of cases submitted to the multidisciplinary team meeting. In many patients, however, there was a lengthy delay between diagnosis and surgery due to late referral, and 13% of our patients were classified as functional class IV at the time of surgery.
The results of our series are excellent, and are comparable to the largest series reported in the literature.21,23 Our success is due to the expertise of our surgeons and the procedural protocol developed by the Multidisciplinary Pulmonary Hypertension Team of the Hospital 12 de Octubre.
The PTE series presented in this study is the largest and most consolidated cohort studied in Spain. In 2009, we published the initial24 results of a series of 30 PTEs. These findings were similar to those published by the Hospital Clìnic de Barcelona25 in a series of 32 patients; in-hospital mortality was 17% and 18.8%, respectively. Both studies found that surgical outcomes improved as the multidisciplinary team acquired more experience. Greater experience is also a factor in improved survival and a gradual increase in the number of patients considered candidates for surgery. In addition, surgical outcomes are better than those of medical treatment. For all these reasons, PTE is the treatment of choice in this disease, and is associated with good outcomes in our hospital.
The accessibility of lesions varies according to the level of experience of the surgical team. In the most experienced teams, the percentage of patients with segmental branch involvement (San Diego group 3) increases over time, although this is not detrimental to outcomes.26–28 Advances in surgical techniques now enable surgeons to dissect and extract material at both the segmental and subsegmental level.
Although the exact PVR or right ventricular dysfunction level that would rule out surgery is unknown, surgical risk and prognosis in the long-term worsen with higher PVR (above 1000dynasscm−5).29,30 Although the risk of surgery is increased in these patients, they can still benefit from the intervention.29 We did not rule out surgery in any patient on the basis of high PVR or severe ventricular dysfunction. In this study, PVR greater than 1000dynasscm−5 was a risk factor for in-hospital mortality in the univariate analysis, but not in the multivariate analysis, even though it is a major risk factor for perfusion injury.
The standard surgical approach to PTE is based on the protocol created by the San Diego group,4 namely, endarterectomy in circulatory arrest with deep hypothermia. This technique has proved both safe and effective. In their most recent series,27 with more than 2700 cases, the group reported a mortality rate of 2.2% in the last 500 patients operated. Although a number of studies have described PTE without circulatory arrest, or at least, with continuous cerebral perfusion,31–34 these techniques did not improve outcomes or diminish neurological complications. All studies in this field have mentioned the difficulty involved in reaching distal branches in the absence of circulatory arrest. In our series, only 1 transient neurological complication occurred, and this was not solely due to circulatory arrest.
Reperfusion injury is inherent to PTE. Incidence ranges from 5% and 20% of all interventions,23 according to different series, and it is a risk factor for in-hospital mortality. This was also found to be true in our series. Manifestation of reperfusion injury varies from mild hypoxia with insignificant radiological findings to severe respiratory failure, similar to respiratory distress. For this reason, it is important to establish a definition of reperfusion injury that limits cases to a clinical entity with an impact on the patient's evolution.
Other series have described the need for extracorporeal membrane oxygenation (ECMO) in heart failure secondary to PH or postcardiotomy syndrome, and severe respiratory failure due to pulmonary hemorrhage or edema.35,36 Berman et al.36 reported a survival rate of 57% in these patients; in our series, it was 40%. These patients would have died without this treatment, and for this reason ECMO is a therapeutic step that should be available in all PTE units.15
Post-PTE survival in our series was excellent, and comparable to that obtained in the most major studies.23 Moreover, in most of our patients, functional class improved, pulmonary pressure was normalized, and as a result, right ventricular remodeling occurred.
Diagnosis of persistent PH is widely debated, both in terms of its definition (mPAP>25, 30 or 35mmHg; PVR>400, 450, 500 or 550dynasscm−5),21,30,37 and the right moment to establish a diagnosis. Incidence varies from 6% to 31%, according to the definition used. Our team decided to define persistent PH as PVR>400dynasscm−5, because unlike mPAP, PVR has both a pre- and postoperative prognostic value. As far as the timing of the diagnosis is concerned, in our series it was made during the 6-month follow-up for two reasons: hemodynamic changes immediately following surgery will affect estimates of PVR, and the prevalence of residual PH increases over follow-up37 when the cause is distal vascular disease.
Despite persistence of PH, this subgroup showed good survival rates and improved functional class, possibly because PVR decreased significantly even in patients with PH. The findings of other groups, such as Freed et al. in Papworth Hospital30 (Cambridge) are largely consistent in terms of patients with residual PH.
ConclusionsOur study contributes further evidence that PTE is the treatment of choice in CTEPH,10 and can be performed successfully in Spain. This surgical intervention has a positive impact on disease prognosis and long-term survival. It also resolves pulmonary hypertension and improves the functional status of most patients.
LimitationsThis is a retrospective, observational study in the initial series of a single hospital.
No information was available on the evolution of NO-pro-BNP markers in patients operated prior to 2009.
One-year follow-up results were analyzed in the 85 patients that reached this stage of the study. Of these, we obtained 76 catheterizations; the remaining 9 were patients that had been referred to their local hospitals, and this information was not forwarded to our group.
AuthorshipLópez Gude MJ and Cortina Romero JM collected data, performed the statistical analysis, interpreted the results and drafted the manuscript.
Pérez de la Sota E, Forteza Gil A and Centeno Rodríguez J performed the surgical procedures.
Eixeres A collected data.
Ruiz Cano MJ, Velázquez MT, Gómez Sánchez MA, Pérez Vela JL, and Sánchez Nistal MA established diagnosis and treated the patients in the clinical setting.
Escribano Subías P established diagnosis and treated the patients in the clinical setting, interpreted the results and drafted the manuscript.
All the authors have reviewed the manuscript.
Conflict of InterestThe authors declare they have no conflict of interest.
Our thanks to the staff of the Hospital Universitario 12 de Octubre Multidisciplinary Pulmonary Hypertension Unit, the medical and nursing staff of the Cardiology Intensive Care Unit, and the medical and nursing staff of the Cardiac Surgery operating theater.
Please cite this article as: López Gude MJ, Pérez de la Sota E, Forteza Gil A, Centeno Rodríguez J, Eixerés A, Velázquez MT, et al. Tromboendarterectomía pulmonar en 106 pacientes con hipertensión pulmonar tromboembólica crónica. Arch Bronconeumol. 2015;51:502–508.