Pulmonary actinomycosis is a very rare diagnosis. It is a chronic, non-contagious, suppurative infection caused by microorganisms from the genus Actinomyces. These are Gram-positive, filamentous, branched bacilli.1 The most common etiologic agent is Actinomyces israelii. Eight species have been identified as pathogenic in humans, one of which is Actinomyces odontolyticus, which very rarely causes lung infections. Pulmonary actinomycosis is mainly caused by the aspiration of these organisms from the oral cavity. We report the case of a patient with severe asthma and chronic eosinophilic pneumonia (CEP), with a diagnosis of pulmonary actinomycosis.
This was a 43-year-old man with severe asthma who had been admitted several times for pulmonary infiltrates that were classified as CEP (diagnosed from bronchoalveolar lavage [BAL] containing 52% eosinophils). He had been receiving oral corticosteroids for a year, but relapsed when the dose was reduced to 10mg/day. Hypereosinophilic syndrome was ruled out by blood tests. Treatment began with omalizumab to reduce the corticosteroid burden, given the patient's significant allergic component (IgE 1089KU/l). Persistent peripheral eosinophilia (peak: 50.3% – 11300mm3) and repeatedly negative ANCA were observed in follow-up clinical laboratory tests. The patient responded well to omalizumab treatment, with no recurrence of asthma or pulmonary infiltrates, and the corticosteroids could be gradually tapered until they were discontinued.
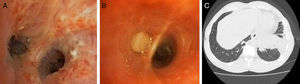
Eighteen months after starting omalizumab and 12 months after discontinuing corticosteroid treatment, the patient consulted due to catarrh and dyspnea. Chest X-ray revealed pulmonary infiltrates and a small left pleural effusion. Of note on clinical laboratory tests was eosinophilia (38% – 3880mm3). Bronchoscopy was performed, revealing mucosa with raised granular-like lesions, showing erythematous spots on a whitish base in the segments. Biopsies were obtained from these lesions (Fig. 1A). The biopsy specimens showed mucosa with squamous metaplasia and intense acute and chronic inflammatory infiltrate in the corium. The BAL cell count found 0% eosinophils. Another observation on bronchoscopy was the left segment 8 blocked by a very dense, whitish, stringy material, which was also biopsied (Fig. 1B). In view of these findings, a chest computed tomography was requested, which revealed an infiltrate in the left segment 8 (Fig. 1C), corresponding with the altered endoscopic image, a biopsy specimen of which subsequently grew A. odontolyticus on culture. The microorganism was identified using MALDI-TOF (matrix-assisted laser desorption/ionization) mass spectrometry, with a score of 1976. Given these results, treatment began with amoxicillin-clavulanic acid for 6 months, along with corticosteroids (1mg/kg). The patient remained stable, without exacerbations and chest X-ray 2 months after admission showed resolution of the infiltrate.
(A) Bronchoscopy: bronchial carina with irregular thickening and mucosa revealing mucosa with raised granular-like lesions, showing erythematous spots on a whitish base. (B) Bronchoscopy: endoscopic image of left segment 8 with dense, whitish material completely obstructing the bronchial lumen. (C) Computed tomography: infiltrate of alveolar aspect with air bronchogram, located in the left lower lobe.
Two months after completing the antibiotic treatment, the patient developed central chest tightness and dyspnea. Chest X-ray was performed, showing a new infiltrate in the left lower lobe. Bronchoscopy was repeated; the same lesions observed previously were present, and biopsies were obtained once again. On this occasion, the biopsy specimen showed intense, predominantly eosinophilic inflammation, and the BAL again showed 0% eosinophils. A. ondontolyticus also grew on culture. A 1-month course of ceftriaxone was administered, followed by amoxicillin for 1 year. In view of eosinophils in blood (35% – 2860mm3) and intense eosinophilic infiltrate in the bronchial biopsy, another cycle of corticosteroids was initiated. The patient's clinical progress was good, and he continues to be monitored in our clinic.
A. odontolyticus is a normal commensal of the oral, gastrointestinal and female genital flora of healthy individuals.2 Predisposing factors for developing the disease are chronic alcoholism, poor oral hygiene, diabetes mellitus, and advanced tumor disease. It is not an opportunistic agent, although immunocompromised hosts are more susceptible.3 Although our case did not present any of the above-mentioned risk factors, he did have a history of prolonged treatment with corticosteroids.
Improvements in general and dental health have led to a reduction in the rate of pulmonary actinomycosis, so it is not taken into account in the differential diagnosis of chronic pulmonary infiltrates. Diagnosis is difficult, and an unknown number of cases remain undiagnosed or are confused with pneumonias, cancer and tuberculosis. Sputum culture is a low-yield procedure for diagnosis, since A. odontolyticus is a habitual contaminant of the oropharyngeal flora, and moreover, it is a slow-growing microorganism that is difficult to culture and requires enriched growth media in anaerobic conditions. Diagnosis in most patients is based on the histopathological identification of the organism in a biopsy obtained by bronchoscopy, transthoracic needle biopsy, or even surgical resection.4
Only one case has been described in the literature in a patient with severe, corticosteroid-dependent asthma, who developed pulmonary actinomycosis during treatment with omalizumab.5 At present, there is no evidence of any association between actinomycosis and eosinophilia. However, some studies suggest that patients receiving anti-IgE develop a Th2 response to Actinomyces. This patient, unlike ours, reported asthenia, anorexia and weight loss, so in view of persistent infiltrate that did not respond to antibiotics and squamous metaplasia in the biopsy, a lobectomy was performed. Omalizumab has been shown to be safe. The development of pulmonary actinomycosis in our patient is probably related to the fact that, although he had not been taking corticosteroids for a year, Actinomyces is slow-growing, so it had most probably been developing since then.
The first-line antibiotic is penicillin G. The recommendation is to start with high intravenous doses for 4–6 weeks, followed by 6–12 months of oral treatment.6
Although A. odontolyticus pulmonary actinomycosis is a very uncommon diagnosis, it should be taken into account in the differential diagnosis of lung disease with recurrent pulmonary infiltrates,4 even in patients who are not immunocompromised.
Please cite this article as: Erro Iribarren M, Cisneros Serrano C, Rajas Naranjo O, García Castillo E. Actinomicosis pulmonar en paciente con neumonía eosinófila crónica en tratamiento con omalizumab. Arch Bronconeumol. 2018;54:51–52.