Introduction
Bolivia forms part of the group of American countries with the highest rate of new tuberculosis (TB) cases (>85/100 000 per year).1 A joint study by the International Union against Tuberculosis and Lung Disease (IUATLD) and the World Health Organization (WHO) established that, out of all the Latin American countries, Bolivia had the second highest prevalence of primary resistance to anti-TB drugs from 1994 through 1997.2
The aim of the present study was to evaluate the results of treatment in people suffering from pulmonary TB in the city of Santa Cruz de la Sierra, Bolivia, who were resistant to one anti-TB drug (single drug resistant [SDR]) or to several drugs (polydrug resistant [PDR]); we excluded combined resistance to isoniazid (H) and rifampicin (R) (internationally called multidrug resistant [MDR]), which is the object of another publication.3
Patients and Methodology
The Department of Santa Cruz is in the eastern part of the country and has a tropical climate. For many years it has headed the list for new TB cases and has also had one of the lowest cure rates.4 In Santa Cruz de la Sierra, the capital of the department, there are 2 broncho-pulmonary care centers financed by the local Red Cross and a German institution (Kuratorium Tuberculose in der Welt). These care centers are responsible for the diagnosis and treatment of TB patients living in 2 parts of the city that contain approximately 53% of the population (about 450 000 inhabitants).5 The fact that a sputum sample was taken systematically from each patient with pulmonary TB for culturing and studies of susceptibility to various anti-TB drugs enabled us to identify patients with SDR or PDR forms in the first sample of sputum.
In 1996 we examined the case histories of all patients treated in these centers from 1983 through 1993 whose sputum culture had produced SDR or PDR strains of Myobacterium tuberculosis. For each patient we recorded demographic variables, the results of microbiological analyses, therapeutic regimens, the number of visits made, x-ray observations, treatment outcome, and the number of months of follow up at the center (until cure, end of treatment, death, failure to complete, or transfer).
Microbiology
The normal procedure at the centers is to obtain 3 sputum samples from each patient for microscopic examination and diagnosis and a monthly sample during treatment and to grow a culture on the first sample and repeat the process during treatment every time the microscopic results are negative. Microbes are cultured on Löwenstein-Jensen medium and recoveries of mycobacteria are confirmed by microscopic examination. When a culture is positive with more than 10 colonies present after 6 weeks of incubation, it is tested for susceptibility to H, streptomycin (S), R, ethambutol (E), and protionamide (Pt) using the proportion method.6 The critical concentrations of drugs that differentiate the susceptible bacilli from the resistant ones are as follows: for H, 0.2 µg/mL; for S, 4 µg/mL; for R, 40 µg/mL; for E, 2 µg/mL; and for Pt, 40 µg/mL.
Internal quality control is carried out using the susceptible strain of M tuberculosis H37Rv. External quality control is carried out by the Asklepios Fachkliniken-Institut für Laboratoriumsdiagnostik de Gauting, Germany, where 10% of all the susceptibility studies, chosen at random, are tested systematically. Whenever asked for, microbiological samples are sent for testing to the Bolivian reference laboratory for such cultures (Laboratorio Nacional de Referencia para Micobacterias, La Paz, Bolivia) and to the Pan American Institute for Food Protection and Zoonosis.
Treatment
The therapeutic regimen applied in the centers during the period of study was different from the one used by the Bolivian national program: 1 HRZS/6 H2R2), where Z refers to pyrazinamide. The standard regimen at the care centers was 1 HRZS/1 HRZ/5 HR and the retreatment regimen was 1 HRZSE/6 HRZE; neither of the 2 regimens was taken under supervision. The treatments for resistant forms of TB were not standardized, but rather were prescribed individually. Some second-line anti-TB drugs were available and once the results of the susceptibility studies were known, patients who did not respond to the initial regimen (standard or retreatment) were prescribed at least 2 drugs to which they were not resistant or which they had not previously taken; the length of the treatment was decided in each individual case but was never less than 7 months. As an incentive, patients were given various food products on each visit.
Definitions
When recording the results of treatment, "cured" meant that the patient successfully completed treatment and recorded negative results in 2 consecutive microscopic examinations of sputum samples and cultures, separated by at least 1 month, one of which was obtained during the last month of treatment; "failed to complete" meant the patient stopped attending before completing the treatment and did not come back to the center; "died" meant the patient died during treatment for whatever cause; "completed treatment" meant the patient completed treatment and the last bacteriological results (microscopic examination of sputum sample and culture) were negative but there was no control sample taken for bacteriology in the last month of treatment; "transferred" meant the patient was transferred to another health center and the enrolling care centers did not know the result of the treatment.
In compliance with recent directives from the WHO, the terms primary, secondary, and initial resistance were replaced by "resistance in new patients" and "resistance in previously treated patients."7 A change in the type of resistance was defined as a change in susceptibility to any anti-TB drug during treatment.
Statistical Analysis
Associations among categorical variables were aalyzed using the χ² test and Fisher's exact test. The continuous variables were analyzed by obtaining the means (SD) and medians and applying Student t tests. The null hypothesis was rejected with values of P<.05.
Results
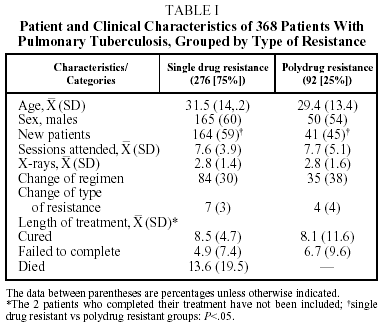
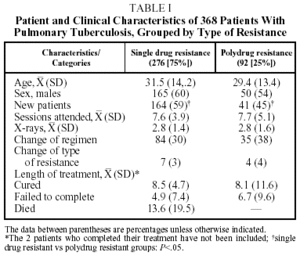
During the study period 368 patients were identified: 276 (75%) were SDR and 92 (25%) were PDR. (One hundred and forty three MDR patients detected during the same period were the object of a separate study; only 28% of them successfully completed the treatment, 48% failed to complete, and 10% died.3) Table 1 shows diverse characteristics of the SDR and PDR groups. The only significant difference between the SDR and PDR groups was in the greater number of new cases in the SDR group (P<.05). There was a slight nonsignificant tendency to change the therapeutic regimen more often in the PDR group (38%) than in the SDR group (30%). The SDR patients whose therapy was not changed were more likely to fail to complete treatment (P<.01) or not be cured (P<.01) than were patients whose treatment was changed. These differences were not found in the PDR group, where changes in therapy always involved at least 3 drugs to which the patient was susceptible. The length of treatment among patients who were cured was similar in both groups, and not much longer than that of nonresistant patients treated at the centers (7 months).
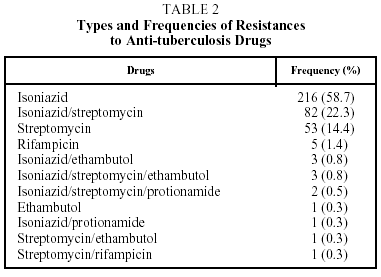
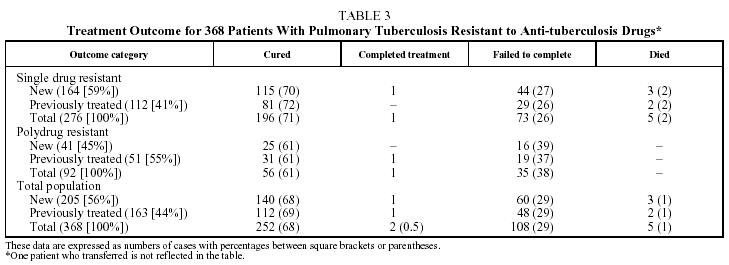
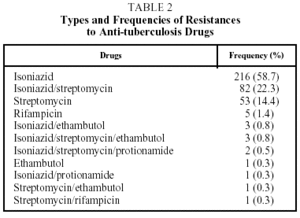
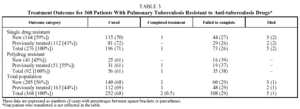
Table 2 shows the different types of resistance and the frequency with which they occurred. Fifty-nine percent of the cases had resistance to H and 95% to H, S, or HS. In a very small number of patients (11 [3%]) a change in the type of resistance was detected during treatment, with no significant differences between SDR and PDR patients. Table 3 shows the results of treatment. The SDR patients were more likely to be cured, but the results were without statistical significance. There were no differences in either group in the cure rate between new and previously treated patients. The percentage of patients who failed to complete treatment was higher in the PDR group than in the SDR group (P=.03). Very few patients died and there were no failures of therapy. Twelve patients (3%) suffered relapses: 7 were cured and 5 failed to complete treatment.
Discussion
Inadequate treatment with anti-TB drugs leads to the appearance of resistant bacilli and this has now become one of the main obstacles to the success of anti-TB therapy.8 These bacilli are the product of the selection of mutant forms that occur naturally in very small quantities in any sizable population of mycobacteria9,10 and whose clinical impact has increased with the elimination of the bacilli that are susceptible to prescribed drugs. This phenomenon was soon detected in the 1940s, shortly after the treatment of pulmonary TB started using streptomycin only.11 Mitchinson12 has proposed 4 mechanisms that might explain this selection of resistant mutations.
The objective of the present study was to establish the prognosis of pulmonary TB patients with any kind of resistance except MDR who live in a city in a very poor country but who receive treatment in an institution with exceptional resources not available to patients in other similar areas: second-line anti-TB drugs and a laboratory that grows cultures and performs susceptibility studies systematically on all patients.
It was not possible to establish the percentage of resistant patients because we did not know the total number of patients identified during the first 3 years of the study (only 5 resistant patients were detected during this period). During the 10 years from 1986 through 1995 the proportion of patients with any kind of resistance among the 3518 new patients was 10% (annual variation: 8%-16%) while among the 957 previously treated patients it was 37% (annual variation: 23%-59%).13
Resistance to H, S, and the HS combination accounted for the great majority (95%) of the resistances detected. This rate is not surprising as these are the longest used anti-TB drugs in Bolivia and it is consistent with the surveys carried out by the WHO and the IUATLD.2
The percentage of new patients that had not been treated previously was very high and, as is to be expected, it was higher among SDR patients (P<.05), a group with what was previously referred to as having primary resistance.7 It must be said, however that ours was a retrospective study and new and previously treated patients may therefore not have been clearly differentiated when a detailed, unhurried initial interview had not been performed. Even when such interviews occurred, however, the fact that a large number of patients had little formal education and only spoke indigenous languages could still have led to errors in classification. We must also take into account that in the area studied there was a certain degree of therapeutic anarchy that might be the cause of a large percentage of the resistances seen in new patients.14
The patients who were cured attended a similar number of sessions, had a similar number of x-rays and their cures took no longer than those of the susceptible patients who attended the same care centers. The cure rate tended to be higher for SDR patients than for PDR patients (nonsignificant, P=.70). These rates are not much lower than the cure rates for the whole population of patients in the region during the period of study (75%)4; in the majority of cases that were not cured the cause was not failure of the therapy but failure to complete the treatment. These results are consistent with those of other authors who have found that resistance to 1 or 2 drugs, not including rifampicin, does not imply a worse prognosis in short therapeutic regimens using 4 drugs, and that initial detection of resistance to H, S, or HS does not necessarily mean changing the length of treatment if the patient shows clinical improvement.15-17
An extensive study carried out in 6 very different parts of the world found significantly lower cure rates in previously treated patients than in new patients, whether susceptible or resistant.18 However, new patients resistant to H (excluding MDR) had a similar cure rate to susceptible new patients (83% compared to 85%). This may be one of the reasons why our study did not detect differences in cure rates between new and previously treated patients (given that 95% of the resistances were to H and/or S).
The limitations of this study should be taken into account. First, as already stated, its retrospective design may have led to errors in classification. Second, as the regimens were not standardized and there were no precise guidelines, it was the attending physician who decided the length of treatment in a way that was not always explicit. Lastly, a greater number of relapses might have been detected if patients had been monitored actively and for longer periods.
In conclusion, cure rates for patients with SDR and PDR pulmonary TB, whether new or previously treated, were not much lower than those of susceptible patients in the same region. A large number of patients in both groups were not cured but this was due to the high rate of failure to complete treatment rather than to failure of the treatment itself. It is unlikely that higher cure rates can be attained in this area unless a form of directly observed treatment using a short-course of antibiotics is used.19
Acknowledgments and Dedications
We are grateful for the help given by Teresa Equisuany in collecting and ordering the data.
We dedicate this article to Dayana Rodríguez, whose mother died of MDR tuberculosis at the age of 18 in Villamontes (Tarija, Bolivia).
Correspondence: Dr. J.E. Ollé Goig.
P.O. Box 3017. Kampala. Uganda.
E-mail: olleuganda@yahoo.com
Manuscript received October 2, 2002.
Accepted for publication March 11, 2003.