Introduction
Community-acquired pneumonia (CAP) is a common infection with high morbidity. The exact incidence of CAP is not known because it is not a reportable disease, but it is estimated that it could range between 1.6 and 13.4 per 1000 people per year.1-4 Since the morbidity and mortality of CAP are high and the disease represents a major cost to health services all over the world, the treatment prescribed should be as effective as possible. The treatment strategies recommended in the guidelines published by different international institutions are, however, controversial and difficult to standardize for all patients and across different geographical areas.5-8 One of the main reasons for modifying the established regimens of empirical therapy is the variation in the susceptibility patterns of the most common pathogens, particularly Streptococcus pneumoniae and Haemophilus influenzae. Relatively few studies have been carried out in Latin America on the etiology of CAP and the patterns of antibiotic susceptibility of its causal agents. In 2 studies carried out in Brazil and Argentina, a high prevalence of atypical pathogens was observed.9,10 It is also worth noting that the scant data available on resistance indicates that the resistance of the pneumococcus to penicillin may be as high as 25%.11
It is important to obtain data on the etiology of CAP and the patterns of antibiotic susceptibility across different geographical areas, as well as information concerning the efficacy of new antibiotics in this context. This paper brings together data from hospitals in 5 Latin American countries which participated in an international clinical trial undertaken to evaluate the efficacy and safety of treatment with moxifloxacin in comparison with amoxicillin, both administered for 10 days, in adult patients with suspected pneumococcal CAP. This paper presents details of the pathogens isolated and the patterns of sensitivity to traditional antibiotics.
Method
Study Design
This was a prospective, multicenter, multinational, controlled, double-blind, randomized study conducted in 82 centers located in 20 countries to compare the efficacy and safety of moxifloxacin and amoxicillin in the treatment of suspected pneumococcal CAP in adult patients. The methodology of this study was recently published elsewhere.12 The present paper describes the pathogens isolated, the patterns of antibiotic sensitivity, and the clinical and microbiological results obtained in the hospitals located in Latin America. The participating countries were Mexico, with 7 hospitals, and Chile, Argentina, Uruguay and Brazil, with 1 each. Patients eligible for inclusion in the study were randomized to receive one of the following treatments during a 10-day period: 400 mg of moxifloxacin (tablets) once a day, or 500 mg of amoxicillin (2 capsules 3 times a day). Patients were either hospitalized or treated as outpatients depending on the criteria of each investigator.
This study was conducted with approval from the ethics committees in each of the participating countries. Informed consent was obtained from each patient prior to entry into the study.
Study Population
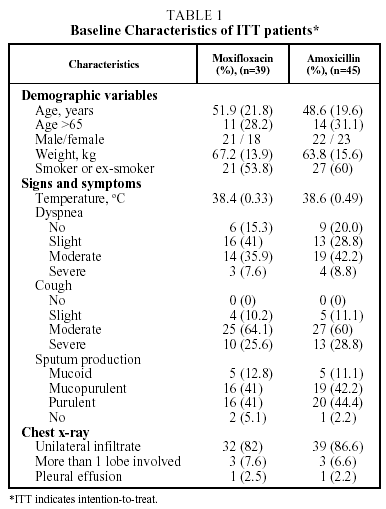
Adult patients (≥18 years of age) presenting with CAP of suspected pneumococcal origin were enrolled in the study. Patients were classified as having CAP if they presented with fever (rectal temperature >38.5ºC or oral temperature >38.0ºC), radiologic evidence of pneumonia, and at least one of the following signs or symptoms: purulent sputum, dyspnea/tachypnea (>20 breaths/min), and/or auscultatory findings indicative of pulmonary consolidation. The etiology was suspected of being pneumococcal if the patient presented with at least 1 of the following findings: rapid onset of symptoms (≤48 hours), high fever (rectal temperature ≥39°C, or oral temperature ≥38.5°C) accompanied by rigors or chills, pleuritic chest pain, chest radiograph showing an infiltrate, or the presence of Gram-positive cocci on direct sputum stain.13,14
Patients were excluded from the study if they presented with a history of hypersensitivity to quinolones or penicillins, prior history of tendinopathy associated with quinolones, or suspected aspiration pneumonia. Patients were not admitted if they had a serious respiratory infection requiring parenteral therapy or mechanical ventilation, a concurrent disease considered likely to interfere with the clinical course of the pneumonia, or neutropenia (neutrophil count <1000 cells/µL). Also excluded were patients suffering from acquired immunodeficiency syndrome who had a CD4 cell count of <200 cells/µL, and those with significant renal impairment (serum creatinine level >3.0 mg/dL [>265 µmol/L]), hepatic disease (baseline alanine aminotransferase or aspartate aminotransferase and/or total bilirubin level more than 3 times the upper limit of normal), serious heart failure, or a life-threatening disease and life expectation of <2 months. Pregnant and lactating women, women in whom pregnancy could not be excluded, and patients hospitalized for >48 hours before the onset of pneumonia were not eligible for entry into the study.
Also excluded were patients who had received a systemic antibiotic to treat the current episode of pneumonia for >24 hours prior to enrollment; patients who had received an experimental drug during the previous 3 months; patients with prolonged QT on an electrocardiogram, or who were being treated with drugs to prolong the QT, such as amiodarone, sotalol, disopyramide, quinine, procainamide, or terfenadine.
Study Protocol
Patients were evaluated on enrollment into the study (visit 1), after 3 to 5 days of treatment (visit 2), and 3 to 5 days (days 1315; visit 3) and 20 to 28 days (days 3138; visit 4) after the end of treatment.
The decision whether or not to hospitalize patients was left to the discretion of the investigator. Adverse events were recorded at every visit, and were classified by the investigator as to their severity and relationship to the medication being studied.
Microbiology
Two blood samples were obtained for cultures from each patient before treatment was started. At visit 1, samples of bronchopulmonary secretions were also collected for culture. A sample of sputum or bronchial material was obtained by 1 of the following methods: bronchoalveolar lavage (BAL) or protected specimen brush in a telescoping catheter, transtracheal aspirate, or pleural fluid by aspiration of the effusion.
Gram's stains were performed on all sputum samples and all bronchial specimens. The quality of the sputum and of the nasotracheal and endotracheal aspirates was evaluated cytologically using a low power lens (100x) and only samples with >25 leukocytes and <10 epithelial cells per field were considered acceptable for culture. In a mixed colony, a count of ≥106 colony forming units (cfu) per mL was required to distinguish the microorganisms considered to be causing the pneumonia from contaminants. The quantitative cut point for the evaluation of the cultures obtained by expectoration and transtracheal aspirate was 106 cfu/mL. The cut points for BAL and protected brush specimens were 104 cfu/mL and 103 cfu/mL, respectively.
Antibiotic susceptibility (minimum inhibitory concentration values [MIC]-90) was determined for Gram-positive and Gram-negative pathogens using the E-test (AB biodisk: Solna, Sweden) for both moxifloxacin and amoxicillin as well as for clarithromycin, cefuroxime, and clavulanic amoxicillin-acid.
Blood and urine samples were collected at visit 1 for detection of the pneumococcal antigen by counterimmuno-electrophoresis. Blood samples for serological testing for Legionella pneumophila, Mycoplasma pneumoniae, Chlamydia pneumoniae, Chlamydia psittaci and Coxiella burnetii were collected at visits 1 and 4. All the serological tests were carried out at a centralized reference laboratory (Glarif Cerba, Baillet en France, France).
Efficacy Analysis
All the patients who were enrolled in the study and received at least 1 dose of the study medication were evaluated as patients with intention to treat (ITT population). Patients who were given the study medication for a minimum of 48 hours (in the case of clinical failure) or 5 full days (in the case of clinical success) were included in the evaluable population (EP). Efficacy analyses were performed on both populations.
The primary efficacy variable was clinical response 3 to 5 days after completion of treatment (visit 3). The patient was considered to be cured when the acute signs and symptoms related to the infection had disappeared, or had improved so much that the patient no longer required additional or alternative antibiotic therapy. Treatment was considered a failure when there was an insufficient reduction of the signs and symptoms of infection such that the patient required additional or alternative antibiotic therapy or died as a consequence of the primary diagnosis (pneumonia). The outcome was classified as undetermined when clinical assessment was not possible (premature withdrawal after less than 2 days of treatment, patient unavailable for evaluation, etc). The early failures (at visit 2) were also classified as failures at visits 3 and 4, and the failures at visit 3 were also considered failures at visit 4.
Clinical success was defined as cure in both the evaluable and the ITT populations. Clinical failure in the EP was defined as a failure or relapse (initial or partial resolution of clinical signs and symptoms during the study, but with subsequent recurrence of the clinical condition making further antibiotic therapy necessary within 21 to 28 days after the period of administration of the study drug). Clinical failure in the ITT population was defined as failure/relapse, undetermined, or missing efficacy data.
Patients for whom at least 1 pathogen was identified in an acceptable pretreatment culture and who had a valid post-treatment bacteriological evaluation were included in the population of microbiologically valid patients. Infection was also considered to be documented when a pathogen was detected in blood culture, or the pneumococcal antigen was found in the patient's serum or urine, especially if this was associated with a positive culture. In the case of atypical pathogens, infection was considered present if there was a fourfold rise in antibody titers or a value of >64 in the case of L pneumophila, or a single titer immunoglobulin M of >8 for M pneumoniae using immunofluorescence assay, immunoglobulin G of >128 for L pneumophila and C Pneumoniae, or >64 for M pneumoniae.
The bacteriological response was classified as eradication (initial pathogen absent during or after treatment), presumed eradication (sampling rendered impossible owing to clinical improvements which made the production of sputum impossible), persistence (repeat isolation of the pathogen during or after completion of treatment), presumed persistence (clinical failure without control culture) or superinfection (isolation of a new pathogen during or after completion of treatment, associated with a recurrence of the clinical signs and a new radiologic infiltrate).
Bacteriological success at the end of treatment (visit 3) and on follow up (visit 4) was defined as eradication or presumed eradication. Bacteriological failure at visit 3 was defined as persistence, presumed persistence or superinfection and at visit 4 as persistence, presumed persistence, eradication with reinfection (eradication of the initial causal pathogen at visit 3, but with isolation of a new pathogen before visit 4 associated with a clinical relapse) or eradication with recurrence (eradication of the initial causal pathogen on visit 3, but isolation of the same pathogen before or at visit 4 associated with clinical relapse).
Safety Analysis
All the randomized patients who received at least 1 dose of the study medication were evaluated in the safety analysis. Safety evaluations were carried out throughout the whole study period (from visit 1 to visit 4).
Results
Patients
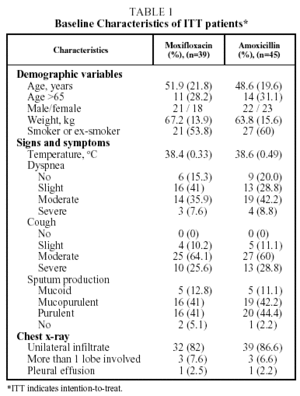
A total of 84 patients were included in the study. They came from 5 participating Latin American countries. Of these 70 (83.3%) could be evaluated for efficacy and safety at the end of the treatment (visit 3); 34 of the group treated with moxifloxacin and 36 of the group treated with amoxicillin. A total of 37 patients (52.8%) were hospitalized; 17 in the moxifloxacin group and 20 in the amoxicillin group. The characteristics of the patients are given in Table 1.
Identification of Pathogens
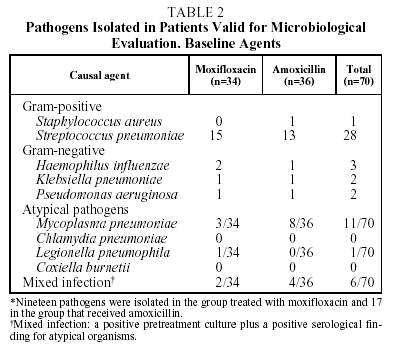
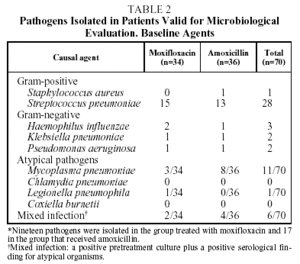
Causal organisms were cultured pretreatment in 36 of the 70 patients evaluated (51.4%); 19 in the group treated with moxifloxacin, and 17 in the group treated with amoxicillin. Gram-positive pathogens were cultured in samples from 29 patients (80.5%). S pneumoniae was detected in the samples of 28 patients (77.7%). Gram-negative pathogens were cultured in samples from 7 patients (19.4%), the most commonly isolated pathogen being H influenzae in 3 patients (8.3%). No pathogens were isolated in blood cultures (Table 2).
The serological determinations were positive for atypical microorganisms in 18 of the 70 patients evaluated (25%), mainly M pneumoniae (n=11).
Mixed infections were found in 6 patients (8.5%). This was defined as the presence of a positive serology for atypical microorganisms and pretreatment isolation of a causal agent in a positive culture.
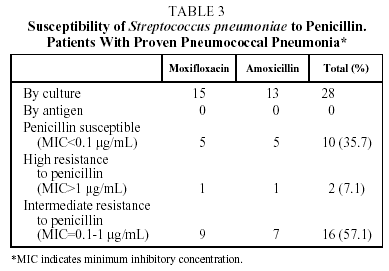
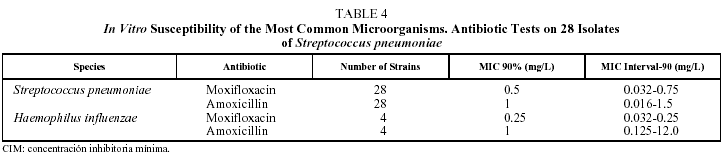
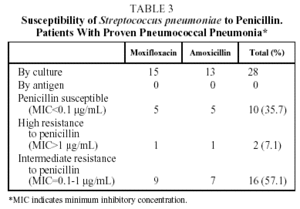
With respect to pneumococcal etiology, 28 of the 70 patients evaluated (40%) had proven pneumococcal pneumonia, demonstrated by positive sputum culture in 27 patients and by isolation in BAL in 1 case. The susceptibility to penicillin of these 28 strains of S pneumoniae was tested. With the breakpoints traditionally used to ascertain susceptibility to penicillin, 10 strains (35.7%) were shown to be susceptible to penicillin (MIC<0.1 µg/mL), 2 (7.1%) presented high resistance to penicillin (MIC>1 µg/mL) and 16 (57.1%), intermediate resistance (MIC between 0.1 and 1 µg/mL) (Tables 3 and 4). However, no strain of S pneumoniae presented resistance to penicillin as defined by the new criteria of the National Committee for Clinical Laboratory Standards (NCCLS) (MIC>4 µg/mL).15 The initial bacteriological findings were comparable in the 2 treatment groups. With respect to macrolides, 4 strains (14.3%) had an MIC>2 µg/mL for clarithromycin and 1 of them an MIC>8 µg/mL. No differences were observed between the hospitalized patients and the outpatients in the percentage of strains with decreased susceptibility to antibiotics.
Efficacy
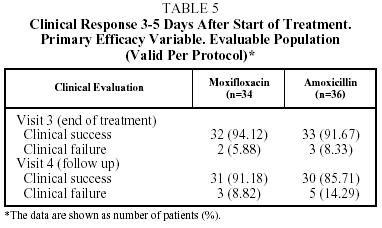
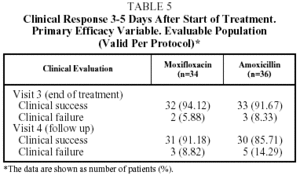
The clinical success rate in the EP at visit 3 (end of treatment) was 94.1% for moxifloxacin and 91.7% for amoxicillin, and at visit 4 (the follow-up visit) this figure was 91.2% for moxifloxacin and 85.7% for amoxicillin. In the ITT population the clinical success rate at visit 3 was 91.9% of the patients treated with moxifloxacin and 85.4% of those treated with amoxicillin, and at the follow-up visit, 89.2% for moxifloxacin and 84.2% for amoxicillin (Table 5).
In the EP, 2 of the 34 patients in the group treated with moxifloxacin (5.9%) and 3 of the 36 patients in the group treated with amoxicillin (8.3%) were considered clinical failures at the end-of-treatment visit (visit 3). In the 2 treated with moxifloxacin no pathogen was isolated; however in the 3 treated with amoxicillin, pathogens were isolated (S epidermis [n=1] and H influenzae [n=1]).
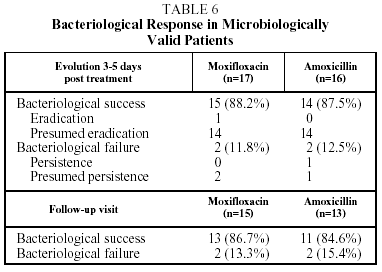
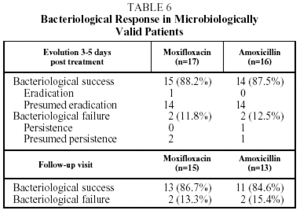
The bacteriological success rate in the EP at the end of treatment and on follow up (visit 4) was 86.6% in the group treated with moxifloxacin and 84.6% in t he group treated with amoxicillin. The bacteriological success rate at the end of treatment (visit 3) was 88.2% for moxifloxacin and 87.5% for amoxicillin (Table 6).
Bacteriological failure (persistence, presumed persistence, or persistence with superinfection) at the end of treatment and after follow up occurred in 11.8% and 13.3% respectively, in the group treated with moxifloxacin and in 12.5% and 15.3% respectively in the group treated with amoxicillin. The sample size in the Latin American arm of the study does not have sufficient statistical power to allow for comparisons of efficacy between the 2 treatment groups. The comparison for the study as a whole has been described recently.12
The pretreatment MIC values for S pneumoniae ranged between 0.032 and 0.75 mg/L for moxifloxacin and from 0.016 to 1.5 mg/L for amoxicillin. In the cases of H influenzae they ranged from 0.032 to 0.25 mg/L for moxifloxacin and from 0.125 to 12 mg/L for amoxicillin.
Safety
During treatment adverse events considered by the investigator to be related (possibly or probably) to the study medication occurred in 27 of the 39 patients (69.2%) in the group treated with moxifloxacin and in 20 of the 45 patients (44.4%) in the group treated with amoxicillin.
The most frequently recorded adverse events were gastrointestinal disturbances (nausea, vomiting and diarrhea) in the group treated with moxifloxacin and liver function test abnormalities in the amoxicillin group.
Drug-related adverse events in both treatment groups were mainly mild to moderate in intensity and were subsequently resolved.
Severe drug-related adverse events were observed in 2 patients in the group treated with amoxicillin (pulmonary embolism and pneumonia relapse) and in 1 patient in the group treated with moxifloxacin (myocardial ischemia with ventricular fibrillation). Twelve patients discontinued the study medication owing to an adverse event, 4 in the group treated with moxifloxacin and 8 in the group treated with amoxicillin. Two patients, 1 from each group, died during the study. Neither of these deaths was considered to be related to the study drug treatment.
Discussion
The results of this study reveal a high prevalence of S pneumoniae with reduced susceptibility to penicillin in patients with CAP in Latin America. Moreover, 8.6% of the patients presented mixed infection. These results are relevant to the orientation of empirical treatment of CAP in Latin America.
One of the main reasons why the treatment of CAP continues to be a challenge for doctors is the large number of causal organisms and the changing patterns of their susceptibility to different antibiotics. Antibiotic treatment for CAP should be active against the most commonly isolated pathogens and, above all, against S pneumoniae. A growing problem recently has been the appearance of strains of S pneumoniae whose resistance to penicillin is not mediated by beta-lactamase. This resistance of the pneumococcus to penicillin is a worldwide problem that has been increasing in recent years to different degrees in different geographical areas. The highest levels of resistance in Europe have been identified in countries where they account for 50% of the isolations: France, Hungary, Spain, Portugal, and Iceland.16-25 In the USA, the level of resistant strains went from 3% in 1988 to 32% in 1998.26 In Latin America, penicillin resistance is estimated to be around 25%, although this is mainly intermediate resistance, with an MIC between 0.12 and 1 µg/mL.11
Our results confirm these high levels of penicillin resistance. Of the 28 strains of pneumococcus isolated, 10 (35.7%) were shown to be susceptible to penicillin, and 18 (64.3%) resistant; 2 strains (7.1%) evidenced high resistance to penicillin and 16 (57.1%) intermediate resistance. It should be noted that this in vitro resistance does not correspond directly with clinical response,27 so that the NCCLS changed the breakpoints for susceptibility to penicillin to 4 µg/mL in 2002.15 None of the pneumococcus isolated in this study had an MIC of >4 µg/mL.
Penicillin resistance also occasionally implies cross-resistance with other antibiotics, such as macrolides, sulfamides, and cephalosporins, so that the activity of the new macrolides, such as clarithromycin or azithromycin, against the pneumococcus is often weaker against the penicillin resistant strains.
The increase in the patterns of resistance of most of the organisms that cause CAP makes it necessary to search for new antimicrobial agents that can be administered empirically. The number of available therapeutic options has increased thanks to the recent development of the fluoroquinolones, whose activity against S pneumoniae is not affected by resistance to penicillin or macrolides.28,29 The new fluoroquinolones with action against the pneumococcus maintain their activity against S pneumoniae, even against strains highly resistant to penicillin. Within this group moxifloxacin is the drug that presents the greatest activity in vitro against the pneumoccus.28
Most etiology studies show that S pneumoniae continues to be the main cause of CAP. The incidence of other pathogens varies geographically and according to the season.3,30-34 The other causal agents in order of frequency are L pneumophila,31Klebsiella pneumoniae32 and H influenzae.32 Occasionally viral agents and C pneumoniae have been isolated with greater frequency than S pneumoniae, especially in the individuals treated as outpatients.34,35
Few studies have been conducted to study the etiology of CAP in Latin America. In a study carried out in Brazil it was observed that "atypical agents," including Chlamydia sp, either alone or in mixed infections can be detected in around 50% of cases when serological techniques are used.9
It should be remembered that the diagnostic tests have limitations, such that the etiology is unknown in 30% to 50% of CAP patients, although some studies show that most cases in which no etiological diagnosis is reached are due to S pneumoniae.36,37
The number of patients with microbiologically valid specimens for our study was 36/84 (42.8%). The pathogens most frequently detected were S pneumoniae (78%), followed in frequency by H influenzae (8.3%) and by M pneumoniae in third place.
The role of the "atypical" pathogens is very controversial, given that their frequency as causal agents of CAP depends on the diagnostic tests and criteria used. The term "atypical" is falling into disuse because the clinical syndrome caused by these microorganisms is not distinctive, but it can still be used to refer to a group of microorganisms (M pneumoniae, C pneumoniae and Legionella spp) rather than to a clinical picture. What is more important is that these are in many cases intracellular organisms, a characteristic that limits the activity of some antimicrobial agents, such as the beta-lactams, and the possibility of their causing mixed infection with classical bacteria with a frequency that ranges between 10% and 40% of cases according to studies undertaken.31,38,39 It is difficult to estimate how important these atypical pathogens are, and whether they require specific treatment. However, various studies have shown that in both hospitalized patients and outpatients the clinical course is less complicated when macrolides are used as part of the therapeutic regimen or when a quinolone is used alone.40-42
The serological determinations were positive for atypical microorganisms in 25% of the EP, above all M pneumoniae and L pneumophila. Mixed infections were found in 6 patients (8.5%), (defined as the presence of positive serology for atypical microorganisms and the isolation by positive pretreatment culture of a causal agent.
Studies designed to investigate the etiology of CAP and its resistance patterns are essential for the orientation of empirical therapy. The new quinolones, such as moxifloxacin, have been shown to be at least as effective as the reference antibiotics in the treatment of CAP.12,13,43
*The names of the other members of the CAP 5 Latin American Study Group are shown in the appendix.
This study was financed by Bayer AG.
Appendix
The members of the CAP 5 Latin American study group are as follows: Abel Jasovich (Argentina); José R. Jardim (Brazil), Marcelo Wolff (Chile), José Alva, Ariel Estrada, Mayra Loera, Juan Morales, Gerardo Rico, Enrique Obispo, and Juan Urueta (Mexico), and Homero Bagnulo (Uruguay).
Correspondence: Dr. M. Miravitlles.
Servicio de Neumología (UVIR, escalera 2, planta 3). Hospital Clínic.
Villarroel, 170. 08036 Barcelona. Spain.
E-mail: marcm@clinic.ub.es
Manuscript received September 30, 2002. Accepted for publication April 29, 2003.