Alpha-1 antitrypsin deficiency (AATD) is still underdiagnosed, despite the recommendation to determine AAT in patients with chronic obstructive pulmonary disease (COPD).
ObjectiveTo estimate the prevalence of AATD in COPD patients adjusted according to the population of the COPD prevalence study in Argentina (EPOC.AR).
Material and methodsThis was a multicenter prospective cross-sectional study of a population aged ≥ 30 years of age diagnosed with COPD, involving AAT quantification in dry blood spot and subsequent genotyping in subjects with < 1.5 mg/dl AAT in dry blood spot (< 80 mg/dl in serum). AAT was defined as the detection of variants ZZ or SZ on genotyping. The EPOC.AR study population was used to calculate local adjusted prevalence.
ResultsWe included 3254 patients (544 with AAT < 80 mg/dL) with a spirometric diagnosis of COPD. The prevalence of AATD in the total study population was 1.29% (95% CI 0.93–1.74), of which 0.92% (95% CI 0.62–1.31) were Pi*ZZ and 0.37% (95% CI 0.19−0.64) Pi*SZ. The adjusted prevalence of AATD in COPD patients ≥ 40 years of age was 0.83% (95% CI 0.23–2.08). We found that AATD was negatively associated with age (OR 0.94; 95% CI 0.90−0.98; P = .006), smoking habit (OR 0.98; 95% CI 0.96−0.99; P = .009), and FEV1% (OR 0.95; 95% CI 0.91−0.99; P = .015).
ConclusionsThe prevalence of AATD in the adult population with COPD in Argentina is estimated to be 0.83%, which could represent 17,000 cases in our country.
Existe subdiagnóstico del déficit grave de alfa-1 antitripsina (DAAT) a pesar de la recomendación de realizar la determinación de AAT en pacientes con enfermedad pulmonar obstructiva crónica (EPOC).
ObjetivoEstimar la prevalencia de DAAT en pacientes con EPOC ajustada a la población del estudio de prevalencia de EPOC en la Argentina (EPOC.AR).
Material y métodosEstudio prospectivo multicéntrico de corte transversal en población ≥ 30 años de edad con diagnóstico de EPOC. Cuantificación de AAT por toma de sangre capilar en gota seca y posterior genotipificación en aquellos sujetos con concentraciones < 1,5 mg/dl en sangre capilar en gota seca (< 80 mg/dl sérica). Se definió DAAT como la detección de las variantes ZZ o SZ por genotipificación. Se tomó la población del estudio EPOC.AR para calcular la prevalencia local ajustada.
ResultadosSe incluyeron 3.254 pacientes (544 con AAT < 80 mg/dl) con diagnóstico espirométrico de EPOC. La prevalencia de DAAT en la población total del estudio fue de 1,29% (IC 95% 0,93−1,74), de los cuales un 0,92% (IC 95% 0,62−1,31) fueron Pi*ZZ y un 0,37% (IC 95% 0,19−0,64) Pi*SZ. La prevalencia ajustada de DAAT en pacientes con EPOC (≥ 40 años) fue de 0,83% (IC 95% 0,23−2,08). Encontramos asociación negativa de DAAT con la edad (OR 0,94; IC 95% 0,90−0,98; p = 0,006), el consumo de tabaco (OR 0,98; IC 95% 0,96-0,99; p = 0,009) y el VEF1% (OR 0,95; IC 95% 0,91-0,99; p = 0,015).
ConclusionesSe estima que la prevalencia de DAAT en la población adulta con EPOC en Argentina es del 0,83%, lo cual podría representar 17.000 casos en nuestro país.
Alpha-1 antitrypsin (AAT) deficiency is the most common autosomal codominant hereditary disease in adult patients. It manifests as early chronic obstructive pulmonary disease (COPD), liver cirrhosis and, less frequently, paniculitis, systemic vasculitis and other rare diseases.1–3
Severe AAT deficiency (AATD) is usually defined by a serum concentration of less than 35% of the expected value or 50 mg/dl determined by nephelometry2. AATD is associated in more than 95% of cases with Pi*ZZ genotypes and less frequently with other genotypes caused by Z, S, rare and null alleles.1–3
The World Health Organization and international and local guidelines recommend the detection of AAT at least once in the lifetime of all COPD patients, although this disease is generally underdiagnosed.1–7
The prevalence of AATD in the general population ranges from 1:2,000-5,000 individuals in some regions of Europe, to 1:5,000-10,000 in the United States of America and Canada, and is 5 times lower in Latin American countries.1,8 Estimating prevalence in patients with COPD is often limited by being based on an indirect theoretical calculation that does not adjust for population factors or COPD characteristics. For this reason, and because the epidemiological data in Argentina and in the region is so scant, we carried out this study in 2 stages. The first stage, published elsewhere, was to select 1000 patients with COPD, in order to determine the prevalence of AATD by quantification of AAT using dry blood spot (DBS) testing with subsequent rapid genotyping in patients with concentrations below a cut-off threshold.9 The prevalence of AATD in patients with COPD in this study stage was 1.5% (95% CI 0.75–2.25).9 The first stage allowed us to more accurately define the number of patients, adjust the cut-off threshold (from 100 to 80 mg/dl), and improve operational, logistical and cost factors. The second stage of the study, presented and discussed here, included more patients in a multicenter design, who were analyzed along with the first-stage patients. The main objective of the study was to establish the prevalence of AATD using direct adjustment. Our study was based on the population of the EPOC.AR study, which investigated the prevalence of COPD in Argentina during the same period using a representative probabilistic sample of the adult population of our country.10 The secondary objective was to study the characteristics associated with AATD.
MethodsStudy design and populationA multicenter study was conducted in 3 respiratory disease reference centers in the city and province of Buenos Aires which also participated in the EPOC.AR study. The study was prospective, cross-sectional, and included consecutive patients with who consulted spontaneously, ≥ 30 years of age, of both sexes, with a diagnosis of COPD by spirometry (Appendix B See table of centers in supplementary material).
We selected patients with a history of COPD who underwent clinical evaluation, pre- and post-bronchodilator spirometry, and capillary finger stick blood collection to perform AAT quantification in DBS. Patients whose spirometry did not meet COPD criteria or was of insufficient quality, patients whose blood sample was insufficient to perform the determination, and patients who did not present spontaneously due to prior diagnosis or family screening, were excluded. Rapid genotyping was subsequently performed in patients with AAT concentrations below 80 mg/dl.
Definitions and laboratory proceduresPre- and post-bronchodilator spirometry (salbutamol 400 μg) was performed. COPD was determined in patients with a post-bronchodilator FEV1/FVC ratio < 0.7 and the GOLD 2017 classification was used to grade the obstruction.11
Spirometries were performed according to ATS/ERS 2005 standards,12 and the reference equations used corresponded to NHANES III.13
The procedure described in the first study stage 9 was followed, as summarized below. All blood samples from all participating centers were obtained by finger stick blood collection and applied to 5 paper discs (DBS, number 903; Schleicher & Schuell; BioScience Inc., Keene, NH, USA). They were allowed to dry at room temperature before being sent to the central laboratory of the Hospital Italiano de Buenos Aires, where all laboratory determinations were made.
AAT quantification in DBS was performed by immunonephelometry (Immage®; Beckman Coulter, Inc., Brea, CA, USA). A regression curve was constructed to estimate the serum AAT concentration from the concentration in DBS. A cut-off point of 1.5 mg/dl was used, corresponding to 80 mg/dl of serum AAT (Appendix B See supplementary material). Rapid genotyping was performed in patients with AAT levels below the cut-off value, using real-time polymerase chain reaction (PCR) (LightCycler®; Roche Diagnostics, Mannheim, Germany) with primers for amplification of 2 fragments of 177 bp and 229 bp. Specific hybridization probes were used to detect S (E264 V) and Z (E342 K) mutations (Appendix B See supplemental material). Rapid genotyping was performed by real-time PCR in patients with AAT < 80 mg/dl in DBS (n = 544). Genotyping could not be performed in 25 patients (4.6%) for technical reasons related to the sample.
Patients were classified according to the combination of S and Z codominant alleles, and the SZ and ZZ variants were considered to be genotypes associated with severe AAT deficiency (AATD). Serum phenotyping was performed non-systematically and only in selected cases when there was a discrepancy between the protein level deficiency and genotype, and/or a strong clinical suspicion of the investigating physician, and/or to reaffirm the diagnosis.
However, it should be noted that a large proportion of the patients called for phenotyping did not complete the procedure due to various personal, geographical, and economic issues, so this study was not performed in all cases. The isoelectric focusing technique (IEF-Hydrasys ® Sebia, Paris, France) was used for serum phenotyping. In patients with a discrepancy between genotype and phenotype, genomic DNA sequencing from whole blood samples was performed. Amplification of AAT exons 2–5 was performed by PCR with specific primer pairs. Purified PCR products were sequenced using the Applied Biosystems® 3500 Genetic Analyzer (Thermo Fisher Scientific, Foster city, CA, USA). (Appendix B See supplemental material).
Ethical aspectsThe study was approved by the institutional and/or ethical review committees of the participating centers. All patients had to sign informed consent before inclusion. The study was conducted in compliance with local requirements for observational studies (Resolution 1480/2011 of the Argentine Ministry of Health).
Statistical analysisThe sample size was calculated based on an estimated prevalence of AATD of 1.5% of the adult population with COPD, according to values obtained in the interim analysis of the first 1000 patients.9 The calculation was performed with a 95% confidence interval, a design effect of 1.4, and an overall precision of ± 0.5%, so the number of patients diagnosed with COPD confirmed by spirometry and determination of AAT in DBS to be selected was 3,179. Estimating an initial lost-to-follow-up rate of 20%, the number of adult patients with suspected COPD to be selected was 3,740. We estimated the prevalence of AATD in subjects with COPD in Argentina based on the direct adjustment by age, sex, and GOLD 2017 classification of the population of the EPOC.AR study that was obtained by probability cluster sampling10 (Appendix B See supplementary material). To this end, we excluded from this analysis the 52 patients in our cohort who were under 40 years of age, so that it was fully comparable with the EPOC.AR population, which included patients aged 40 and above.
The 95% confidence intervals (95% CI) were calculated using the exact Clopper-Pearson method based on binomial distribution. A bivariate analysis was performed to study variables associated with AATD using non-parametric methods for continuous variables (Kruskal-Wallis) and Chi-squared test (or Fisher's exact) for categorical variables. A logistic regression-adjusted analysis was performed to establish factors possibly associated with AATD, including variables with p < 0.1 in the bivariate analysis in the model. The odds ratio (OR) was used as an association measure, along with the corresponding 95% CI. Significance was set at 5%.
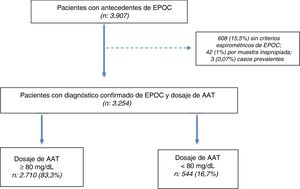
ResultsWe selected 3907 patients aged ≥ 30 years with a history of COPD between July 2009 and July 2018. Of the 3907 patients selected, diagnosis was confirmed in 3254 (83.2%) by post-bronchodilator spirometry, and a DBS sample was obtained for the determination of AAT (Fig. 1).
Population characteristicsOf the 3254 subjects included (total population), 544 (16.7%) had AAT concentrations < 80 mg/dl. The total population was characterized by an average age of 60.9 ± 8.5 years, prevalence of men (64%), high prevalence of current or past smoking (90.6%), with a predominance of severe and very severe disease according to the GOLD 2017 classification (37.3% and 16.5%, respectively) (Table 1).
Patient characteristics.
| Total population (n = 3254) | AAT ≥ 80 mg/dl (n = 2710) | AAT < 80 mg/dl (n = 544) | p | |
|---|---|---|---|---|
| Age in years, mean ± SD | 60.9 ± 8.5 | 61 ± 8.5 | 60.4 ± 8.9 | 0.16 |
| Male sex, n (%) | 2085 (64) | 1731 (63.8) | 354 (65) | 0.62 |
| Smoking categories, n (%) | ||||
| Former smoker | 1993 (61.2) | 1631 (60.2) | 362 (66.5) | 0.8 |
| Smoker | 957 (29.4) | 802 (29.6) | 155 (28.4) | |
| Never smoker | 160 (4.9) | 133 (4.9) | 27 (4.9) | |
| Missing data | 144 (4.4) | 144 (5.3) | 0 (0) | |
| Smoking burden, mean ± SD | ||||
| Pack-years | 57.4 ± 36.4 | 57.7 ± 36.8 | 56.2 ± 34.7 | 0.4 |
| Post-BD spirometry, mean ± SD | ||||
| FEV1% predicted | 49.36 ± 19.0 | 49.14 ± 19.11 | 50.48 ± 18.39 | 0.13 |
| FEV1 in liters | 1.39 ± 0.61 | 1.38 ± 0.61 | 1.44 ± 0.62 | 0.03 |
| FEV1/FVC | 50.12 ± 11.44 | 50.05 ± 11.51 | 50.47 ± 11.08 | 0.43 |
| FVC% predicted | 74.44 ± 19.0 | 74.09 ± 19.11 | 76.19 ± 18.34 | 0.02 |
| FVC in liters | 2.73 ± 0.88 | 2.71 ± 0.88 | 2.83 ± 0.90 | 0.01 |
| GOLD 2017 obstruction grade, n (%) | ||||
| GOLD 1 | 240 (7.4) | 204 (7.5) | 36 (6.6) | 0.1 |
| GOLD 2 | 1265 (38.9) | 1036 (38.2) | 229 (42.0) | |
| GOLD 3 | 1213 (37.3) | 1055 (37.1) | 208 (38.2) | |
| GOLD 4 | 536 (16.5) | 465 (17.2) | 71 (13.0) | |
AAT: alpha-1 antitrypsin; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; post-BD: post-bronchodilator; SD: standard deviation.
We found no clinically relevant differences between the subgroups of patients with AAT determined by DBS ≥ 80 mg/dl and < 80 mg/dl (Table 1).
Prevalence of severe alpha-1 antitrypsin deficiencySevere AATD, defined as SZ and ZZ variants, was detected in 42 patients (7.72%; 95% CI 5.62–10.29). By extrapolating this result to the total population of our study, we observed a prevalence of AATD of 42/3254 (1.29%; CI 95% 0.93–1.74) in patients with COPD included in the study, of whom 0.92% (95% CI 0.62–1.31) were Pi*ZZ and 0.37% (95% CI 0.19−0.64) were Pi*SZ (Table 2).
Rapid genotyping characteristics.
| Total population (n = 3254) | AAT < 80 mg/dl (n = 544) | Phenotyping | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Homozygous for Z | 30 (0.92) | 30 (5.51) | 8/30(26.66) |
| Heterozygous for SZ | 12 (0.37) | 12 (2.21) | 6/12 (50) |
| Homozygous for S | 4 (0.18) | 4 (1.1) | 1/4 (25) |
| Heterozygous for S | 55 (1.69) | 55 (10.11) | 14/55 (25.45) |
| Heterozygous for Z | 33 (1.01) | 33 (6.07) | 15/33 (45.45) |
| No S/no Z | 385 (11.83) | 385 (70.77) | 13/385 (3.37) |
| No genotyping | NA | 25 (4.6) | NA |
AAT: alpha-1 antitrypsin; NA: not applicable.
The table shows the percentages of the different variants in the subgroup with AAT < 80 mg/dl and extrapolation to the total study population. Percentage of cases with genotyping in which phenotyping was also performed (see also Table 3).
In the 15 cases with heterozygous Z genotype in whom phenotyping was performed (by isoelectric focusing), 13 (86.6%) were MZ and 2 (13,3%) were rare MmaltonZ forms, confirmed by sequencing (Table 3).
Characteristics of isoelectric focusing phenotyping performed in selected cases.
| Phenotyping | ZZ n (%) | SZ n (%) | SS n (%) | MS n (%) | MZ n (%) | MmaltonZ n (%) | MM n (%) | Rare M n (%) |
|---|---|---|---|---|---|---|---|---|
| Homozygous for Z (n = 8) | 8 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Heterozygous for SZ (n = 6) | 0 (0) | 6 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Homozygous for S (n = 1) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Heterozygous for S (n = 14) | 0 (0) | 0 (0) | 0 (0) | 14 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Heterozygous for Z (n = 15) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 13 (87) | 2 (13) | 0 (0) | 0 (0) |
| No S/no Z (n = 13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 8 (62) | 2 (38) |
After direct adjustment for age, sex and GOLD 2017 grade of obstructive severity, the prevalence of AATD in patients with COPD (≥ 40 years) in Argentina was 0.83% (95% CI 0.23–2.08).
Adjusted analysis of factors associated with AATDOne of the secondary objectives of the study was to analyze potential differences in demographic and spirometric characteristics in patients with rapid genotyping by comparing patients categorized as no deficiency (no S, no Z), mild to moderate deficiency (heterozygous for S or Z or homozygous for S), and severe deficiency (SZ and ZZ).
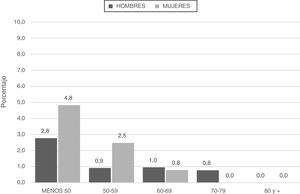
Patients categorized with severe deficiencies (SZ or ZZ) were younger and smoked less in terms of pack-years (Fig. 2; Table 4).
Characteristics of subgroups categorized by rapid genotyping.
| No deficiencya (n = 385) | Mild to moderateb (n = 92) | Severec (n = 44) | p | |
|---|---|---|---|---|
| Age in years, mean ± SD | 60.8 ± 9.01 | 60.1 ± 8.35 | 55.8 ± 9.46 | 0.006 |
| Male sex, n (%) | 252 (65.5) | 64 (69.6) | 22 (52.4) | 0.14 |
| Smoking categories, n (%) | ||||
| Former smoker | 252 (65.5) | 59 (64.1) | 31 (73.8) | |
| Smoker | 113 (29.4) | 30 (32.6) | 7 (16.7) | 0.33d |
| Never smoker | 20 (5.2) | 3 (3.3) | 4 (9.5) | |
| Smoking burden, mean ± SD | ||||
| Pack-years | 56.8 ± 34.1 | 61.6 ± 36.3 | 38.3 ± 36.0 | < 0.001 |
| Post-BD spirometry, mean ± SD | ||||
| FEV1% predicted | 51.22 ± 17.79 | 51.00 ± 20.16 | 42.73 ± 18.25 | 0.011 |
| FEV1 in liters | 1.45 ± 0.60 | 1.52 ± 0.68 | 1.28 ± 0.70 | 0.043 |
| GOLD 2017 obstruction grade, n (%) | ||||
| GOLD 1 | 23 (6.0) | 9 (9.8) | 2 (4.8) | |
| GOLD 2 | 177 (46) | 33 (35.9) | 12 (28.6) | 0.053e |
| GOLD 3 | 143 (37.1) | 34 (37.0) | 18 (42.9) | |
| GOLD 4 | 42 (10.9) | 16 (17.4) | 10 (23.8) | |
| AAT determination, mean ± SD | ||||
| DBS | 63 ± 14 | 51 ± 18 | 29 ± 20 | < 0.001 |
AAT: alpha-1 antitrypsin; DBS: dry blood spot; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; post-BD: post-bronchodilator; SD: standard deviation.
Analysis of the spirometric characteristics revealed that patients with severe deficiencies (SZ or ZZ) were characterized by lower FEV1 and a greater tendency to present GOLD 2017 grades 3–4 (66.7%) than patients with mild or no deficiency genotypes (54.4% and 48%, respectively) (p = 0.053) (Table 4).
After adjustment with logistic regression, the variables associated negatively and independently with AATD were age (OR 0.94; 95% CI 0.90−0.98; p = 0.006), tobacco consumption measured by number of pack-years (OR 0.98; 95% CI 0.96−0.99; p = 0.009) and FEV1% (OR 0.95; 95% CI 0.91−0.99; p = 0.015). In spite of this, these 3 variables showed a low power of discrimination in the analysis of the area under the ROC curve, which was 0.64 (95% CI 0.55−0.73) for age, 0.69 (95% CI 0.60−0.79) for pack-years, and 0.63 (95% CI 0.54−0.73) for FEV1%.
DiscussionCOPD is a global public health problem and mortality is expected to increase over the next 15 years.14 In addition to smoking, there are other factors that contribute to its development, one of the most important being AATD.1,15
Patients with AATD and the PI*ZZ variant are characterized by AAT concentrations below 15% of normal value and higher mortality, especially in the presence of smoking and FEV1 decline. Patients with the PI*SZ variant and AAT levels of 40% of normal values have an increased risk of developing COPD if they are smokers.1,15 Despite the importance of early diagnosis and clinical recommendations, this disease is widely underdiagnosed.1–7
Our study was conducted according to international and local recommendations using a COPD screening program based on the determination of AAT in DBS samples with further evaluation of patients with concentrations below a cut-off threshold.16,17 We included patients ≥ 30 years of age diagnosed with COPD by spirometry and found 42 patients (1.29%) with AATD, of whom 30 were Pi*ZZ variants (0.92%) and 12 Pi*SZ variants (0.37%). These percentages are in line with the range of prevalences reported in different mega-registries from other countries,18–24 which vary from a frequency of Pi*SZ variants of only 0.2% in Germany19 to 7.3% Pi*ZZ and 1.9% Pi*SZ variants in Italy.18 These values are higher than figures reported in Brazil, where a recent study found 0.64% Pi*ZZ variants and 0.1% Pi*SZ variants.24 Differences in prevalence between the registry studies are significant and certainly multifactorial. One of the most important reasons is related to the different criteria applied in patient selection. The inclusion of pre-existing non-spontaneous or known cases of AATD versus only new, spontaneously presenting cases is a very important factor that can falsely increase the prevalence rate, especially when the program is conducted in or influenced by specialized centers with a high interest in and history of investigating the disease; these sites also coincidentally contribute the greatest number of cases. We excluded all pre-existing non-spontaneous cases whose condition was either known or previously diagnosed, and those were identified through family screening of a non-spontaneous index case.
The studies mentioned also differed in terms of cut-off age for patient inclusion, the characteristics of COPD and/or respiratory disease (patients with asthma, bronchiectasis and other chronic respiratory conditions were also included), and the methodology for sample analysis.3,15,22 We have tried to control these potential biases by using patient selection criteria and by following a methodology for sample analysis based on studies from other countries such as Spain21 that complies with current recommendations.16
Even after controlling for these biases, the population of our study shows significant differences in age, sex, and severity of COPD with respect to the COPD population in Argentina. For this reason, we made a direct adjustment (by age, sex and GOLD severity grades) of the prevalence calculation taking as the baseline population the series of the Argentine EPOC.AR prevalence study obtained by probability cluster sampling in the same period.10 From these calculations, it was estimated that 0.83% of adult patients (≥ 40 years) with COPD in Argentina would have AATD. While the exclusion of pre-existing cases, the unification of inclusion criteria and direct adjustment do not eliminate the biases inherent in the design type, we believe that the prevalence figure is close to the real figure, and consistent with population-based sampling studies and genetic analysis with prevalence calculation based on the Hardy-Weinberg equilibrium.25,26
The cut-off value of less than 80 mg/dl for genotyping was chosen for operational, administrative and cost reasons; however, a cut-off value of 120 mg/dl, as currently recommended, could improve the sensitivity of screening.16 One of the additional limitations of our study is that serum phenotyping was performed non-systematically and only in selected cases, and a large proportion of patients did not attend the second testing visit. Because of this, rare variants that are clinically relevant may have been overlooked. This difficulty could be resolved in the future by implementing phenotyping techniques in DBS, offering a definitive diagnosis from a single sample.
When analyzing possible factors associated with AATD, we found that the variables negatively associated with severe deficiency were age, tobacco use measured by number of pack-years, and FEV1. This finding is consistent with those of other studies in which significant differences or trends were observed in the same variables.22,24,27 As mentioned in the guidelines and highlighted in our study, the presence of COPD in a younger population or in individuals with lower exposure to tobacco smoke or more severe disease with lower FEV1 should increase the degree of clinical suspicion of AATD.
ConclusionsIt is estimated that the prevalence of AATD in the adult COPD population in Argentina is 0.83%, which could represent 17,000 cases in our country. The prevalence observed in our study confirms the theoretical population-based estimate8 and implies a high degree of underdiagnosis of the disease. These findings underline the need to promote campaigns aimed at raising awareness of AATD associated with COPD, to extend the screening program to more centers across the country, and to redouble efforts to set up a national registry of patients with this disease.
FundingThis study was carried out with the financial support of the Tuteur S. A. laboratory in Argentina and the Society of Phthisiology and Pulmonology of the Province of Buenos Aires (STNBA). The funding bodies did not participate in study design, data collection, analysis and interpretation, or the drafting of this paper.
Conflict of interestsGM has received financial assistance from Tuteur S. A. and Grifols S. A. laboratories for attending congresses. MFA has received financial assistance from Tuteur S. A. and Grifols S. A. laboratories for attending congresses. ALE has received financial assistance from the Tuteur S. A. laboratory for the attendance of congresses. PBS has received financial assistance from Teva laboratories for attending congresses. MEF has received financial assistance from Tuteur S. A. and Grifols S. A. laboratories for attending congresses. The other authors state that they have no conflict of interests.
We would like to remember and especially thank the first author, Dr. Guillermo Menga, who was a pioneer and leader in the investigation of alpha-1 antitrypsin deficiency in Latin America and who sadly died while this manuscript was being prepared.
We thank the DAAT.AR study group, the centers and researchers of which are listed below: Hospital Municipal de Rehabilitación Respiratoria María Ferrer: Drs. Guillermo Menga and Martin Fernández and Dra. Melina Girbal; Hospital Especializado de Agudos y Crónicos Dr. Antonio Cetrángolo: Drs. Mariano Fernández Acquier, Orlando Lopez Jové, Wu Yu Feng and Hsueh Yu Tang; Hospital Interzonal Especializado de Agudos y Crónicos San Juan de Dios, de La Plata: Dr. Andres L. Echazarreta, Nurse María del Carmen Sanchez, Drs. Maria Fernanda Curró, Silvana Márquez, Yesica Correa, Miriam Sainz, Ana Luz Sánchez and Dr. Raul Grandi Vega; Hospital Italiano de Buenos Aires: Drs. Patricia B. Sorroche, Maria V. Lorenzon and María S. Saez.
We are especially grateful to Sergio Arias and Gustavo Armando (Instituto Nacional de Enfermedades Respiratorias Dr. Emilio Coni, Santa Fe, Argentina) for providing access to the baseline population of the EPOC-AR study for the calculation of prevalence by direct adjustment. We are also grateful to Raúl Bozzo (IC Projects) for his collaboration in data analysis and the coordination of the publication.
Please cite this article as: Menga G., Acquier M.F., Echazarreta A.L., Sorroche P.B., Lorenzon M.V., Fernández M.E., et al. Prevalencia de déficit de alfa-1 antitripsina en pacientes con EPOC en Argentina. Estudio DAAT.AR. Arch Bronconeumol. 2020;56:571–577.