We report the case of a 78-year-old man, former smoker, with a history of pulmonary tuberculosis treated with left therapeutic pneumothorax and chronic recurrent left empyema, who presented with worsening of his general status in recent months, with dyspnea, weight loss, asthenia, generalized skin dryness, symptoms of right heart failure, and chronic hypercapnic respiratory failure.
Of note on clinical laboratory tests were anemia and mild liver enzyme changes. Chest X-ray revealed a significant increase in previous left pleural effusion with mediastinal shift, so thoracentesis was performed, which yielded cloudy pleural fluid, consistent with exudate, predominantly polynuclear, with low glucose, raised proteins, LDH and ADA, and normal CEA. Cytology was negative for malignant cells and culture was negative for bacteria and mycobacteria.
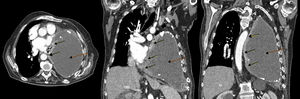
In view of the patient's poor progress after evacuation of pleural fluid and findings suggestive of right heart failure, transthoracic echocardiography was performed, which revealed a hypoechogenic mass in the inferolateral wall of the left ventricle, with dilation of the left atrium, slight pericardial effusion, preserved LVEF, and dilated inferior vena cava without inspiratory collapse. Chest–abdomen computed tomography (CT) was performed, showing a soft tissue mass in the medial portion of the posterior chest wall, measuring 6×3cm, with pericardial infiltration, mass effect, and associated small pericardial effusion. A significant increase in the pleural collection compared to previous studies was visualized, occupying practically the entire hemithorax, causing compressive atelectasis of the lung with contralateral mediastinal shift and cardiac compression (Fig. 1).
Chest CT with contrast medium, axial (left) and coronal slices (center and right): pleural mass in the medial portion of the anterior left chest wall with pericardial infiltration (solid arrows), and a large collection in the left hemithorax, corresponding with chronic pyothorax (dotted arrows), together causing contralateral mediastinal shift and cardiac compression.
A CT-guided biopsy of the mass was performed, which according to the pathology report was consistent with non-Hodgkin's diffuse large B-cell lymphoma, with a proliferation of 70%, no positivity for Epstein Barr virus (EBV) or c-myc, and no bone marrow infiltration. HIV, HBV, HCV and CMV serologies were negative. The patient's clinical situation worsened rapidly in a few weeks, so he was referred for palliative care and follow-up, and died 2 months after diagnosis.
Primary pleural lymphoma is an uncommon entity, accounting for approximately 7% of all lymphomas. It usually affects patients with HIV or chronic pyothorax (CP), and occurs only exceptionally in immunocompetent patients.1 Although the incidence of CP is similar in both sexes, men are more susceptible to developing non-Hodgkin's lymphomas (NHL) than women (ratio of 5.2:1).
Long-term inflammatory stimulation has been identified as an important etiological factor in the development of malignant lymphomas, and studies have reported longer periods between the onset of CP and the development of NHL (>20 years) in these patients compared to patients with autoimmune diseases or renal transplants (9.5 and 4 years, respectively). In fact, Aozasa et al. used the results of their study to differentiate and establish a characteristic clinico-pathological entity called pyothorax-associated lymphoma (PAL). This entity is defined as a B-cell NHL that develops in the pleural cavity of patients with CP of more than 20 years’ standing, in which an exclusive molecular profile has been determined, consisting of overexpression of interferon alpha-inducible protein 27 that plays a role in chronic inflammation.2 EBV causes latent infection in PAL, with type III expression of EBV-related proteins in the tumor cells,3 a phenomenon not observed in our patient.
Although the most common symptom is chest pain, dyspnea may also develop in the presence of significant pleural effusion, as occurred in our case. Radiological signs include diffuse nodular pleural thickening, accompanied by pleural mass. A soft tissue mass in the pleura adjacent to the edge of a coexisting empyema cavity is suggestive of pyothorax-associated lymphoma.4 Isolated pleural effusion may occasionally appear before the pleural mass develops.5 Knowledge of the typical radiological findings and location assists in diagnosing this rare disease.4 In line with the literature, in the months before diagnosis, our patient required repeated evacuating thoracentesis for recurrent empyema, and the pleural mass was only visualized subsequently, located (unusually) in the medial portion of the left posterior chest wall, extending to the pericardium.2
For diagnosis, pleural biopsy should be obtained under ultrasound or CT-guidance or by video-assisted thoracoscopy.5 In histological terms, all cases of PAL are NHL, the most common being diffuse large B-cell type NHL, as found in our patient.5 Aggressive surgical treatment with pleuropneumonectomy is highly effective in early-stage disease, but is therefore only possible in a very small number of patients.3 Systemic chemotherapy based on CHOP combinations is required, but efficacy is variable. Radiation therapy is effective for local and primary control and for rescue therapy after chemotherapy. Prognosis is poor, with a 5-year survival rate of 20%–30%.3 Although this is an uncommon problem, a diagnosis of pleural lymphoma should be taken into account in the long-term follow-up of patients with chronic pleural infection, in order to avoid therapeutic delay.
Please cite this article as: Cerezo-Hernández A, García-Gallardo Sanz MV, Arroyo Domingo CA, del Campo Matías F. Linfoma pleural asociado a empiema crónico. Arch Bronconeumol. 2018;54:400–401.