A topic of interest in chronic obstructive pulmonary disease (COPD) is the identification of biomarkers that can be applied in the clinic. The usefulness of eosinophil counts in blood for predicting future events, such as exacerbations or death, has been under discussion since several cohort studies reported discrepant results.1–6 However, this biomarker predicts the effect of inhaled corticosteroids (ICS), in combination with bronchodilators, in the prevention of exacerbations,7,8 and for this reason, the Spanish COPD guidelines (GesEPOC)9 and the Global Initiative for Chronic Obstructive Lung Disease (GOLD)10 include it in their therapeutic algorithms.
The evidence comes essentially from clinical trials in which the biomarker is determined at the time of recruitment, and the effect of the ICS is evaluated in short-term follow-up. However, these trials do not reproduce clinical practice conditions, and decisions in this setting are often based on historical clinical data. As eosinophil counts can vary over time, and since the optimal cut-off point has not fully been clarified, clinicians face some uncertainty with regard to the use of this marker, particularly if there are discrepancies between the latest and the previous values, or if the clinical laboratory tests were performed some time previously.
For this reason, we designed this study to assess the relationship between eosinophil counts and clinical events (mortality and severe COPD exacerbations) in conditions that reproduce our clinical practice.
We retrospectively reviewed electronic medical records (2009–2018) from a dedicated COPD clinic in a second-level university hospital that included health data from all care levels, providing reliable data on the outcome variables studied. Complete blood counts performed when the patients were stable (>4 weeks from an exacerbation) in the 5 years prior to the index date (first visit) were recorded. The criterion for inclusion was a diagnosis of COPD according to GOLD in smokers of ≥10 pack-years, with a follow-up of ≥12 months. Exclusion criteria were not having ≥3 complete blood counts in the specified period, alpha 1-antitrypsin deficiency, concomitant diagnosis of other chronic respiratory disease, chronic systemic steroid use, and diseases that could alter the eosinophil count. Subjects were divided into 3 groups: (A): eosinophils always <300/μL; (B): variable counts, above and below this value, with less than 3 complete blood counts showing eosinophilia; and (C): at least 3 complete blood counts with ≥300 eosinophils/μL. The relationship between this classification and the risk of death or admission for COPD exacerbations after the index date was studied by constructing Kaplan-Meier curves and analyzing the Cox's proportional hazards model, correcting for confounding variables. An additional analysis was performed dividing persistently eosinopenic subjects (≤100 eosinophils/μL in all complete blood counts) and non-eosinopenic subjects. Receiver operating characteristic (ROC) curves were obtained for the maximum number of eosinophils determined in each patient.
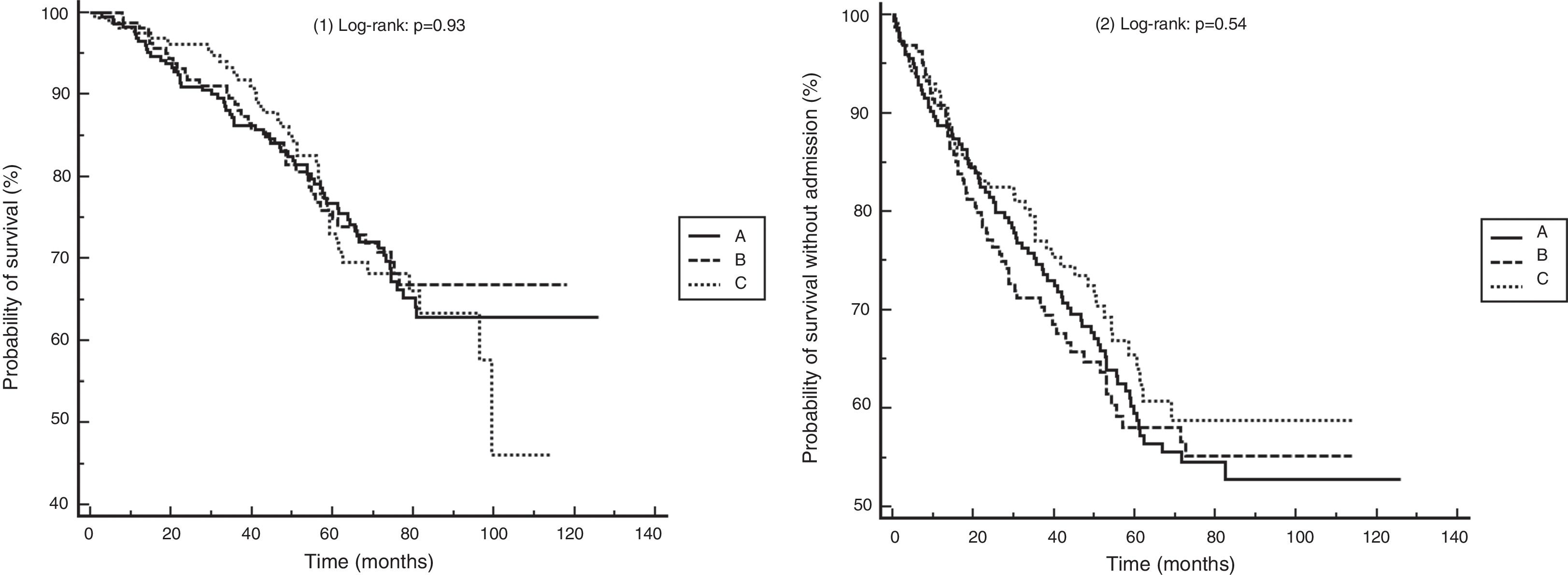
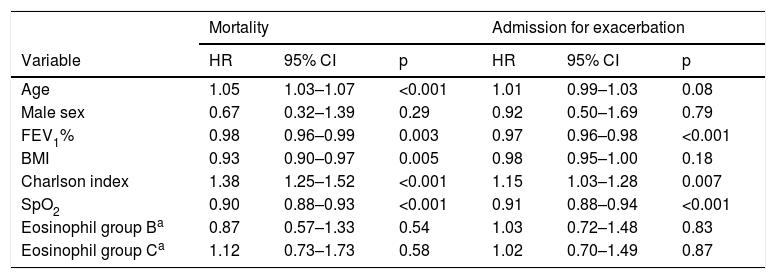
We identified 758 subjects. A total of 204 were excluded (26%), as follows: <3 complete blood counts in stable phase (164), long-term administration of steroids (20), diagnosis of alpha-1 antitrypsin deficiency (15), leukemia (2), and pneumoconiosis (3). We studied 554 subjects with a follow-up of 58.5 ± 26.9 months: Group A: 228 subjects (41.1%); Group B: 165 (29.7%); Group C: 161 (29%). Fifty-two (9.3%) had persistent eosinopenia. There were no differences between groups in terms of age, sex, pack-year index, percentage of active smokers, spirometric values, oxygen saturation, body mass index, comorbidity and BODEx indices, or death rate, nor in the percentage of subjects treated at the index date with ICS or other drugs for COPD. Fig. 1 shows the Kaplan–Meier curves for mortality and the risk of a first admission for COPD exacerbation. Table 1 shows the results of the Cox analysis for both events. Classification using the cut-off point of 300 eosinophils was not associated with differences in the risk of death or admission. Patients with persistent eosinopenia presented a higher risk of death (HR: 1.70; 95% CI: 1.03–2.79; p = 0.03) but not of admission. The area under the ROC curve for the maximum number of eosinophils to predict mortality or hospitalization was 0.51 (95% CI: 0.47–0.56) and 0.50 (95% CI: 0.45–0.54), respectively.
Cox analysis results for risk of mortality and admission for exacerbation.
| Mortality | Admission for exacerbation | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p | HR | 95% CI | p |
| Age | 1.05 | 1.03–1.07 | <0.001 | 1.01 | 0.99–1.03 | 0.08 |
| Male sex | 0.67 | 0.32–1.39 | 0.29 | 0.92 | 0.50–1.69 | 0.79 |
| FEV1% | 0.98 | 0.96–0.99 | 0.003 | 0.97 | 0.96–0.98 | <0.001 |
| BMI | 0.93 | 0.90–0.97 | 0.005 | 0.98 | 0.95–1.00 | 0.18 |
| Charlson index | 1.38 | 1.25–1.52 | <0.001 | 1.15 | 1.03–1.28 | 0.007 |
| SpO2 | 0.90 | 0.88–0.93 | <0.001 | 0.91 | 0.88–0.94 | <0.001 |
| Eosinophil group Ba | 0.87 | 0.57–1.33 | 0.54 | 1.03 | 0.72–1.48 | 0.83 |
| Eosinophil group Ca | 1.12 | 0.73–1.73 | 0.58 | 1.02 | 0.70–1.49 | 0.87 |
BMI: body mass index; SpO2: Oxygen saturation by pulse oximetry.
This study illustrates the difficulties of using the eosinophil count in the clinic. In a quarter of the subjects, no previous complete blood count results (or very few) were available, or elements that might alter results were identified. Only one third have persistent eosinophilia, in line with previous studies.5,11 The maximum interval between obtaining a complete blood count and using the results to guide treatment is not clearly defined in either GOLD or GesEPOC.9,10 Considering that COPD treatment is decided in all areas, including primary care, it is not realistic to expect a recent complete blood count (CBC) in each case, and we believe that clinicians rely on previous historical values that are not always recent. In our study, classification according to historical figures, based on the cut-off point recommended by GesEPOC, was not associated with major clinical events, and the ROC curve for the maximum number of eosinophils indicates that using another cut-off point would not have changed the results. However, patients with persistently very low eosinophil counts had a higher risk of mortality, but this finding that must be interpreted cautiously given the size of the sample. We must recognize that the usefulness of this biomarker lies in predicting the effect of ICS in preventing exacerbations, although this factor could not be judged in this study due to its limitations, since prescription data were only available from the first visit, and not on subsequent developments. In spite of that and other limitations (risk of information bias and clear selection bias due to scope of the study conduct), we believe that our findings underline the advisability of carrying out studies that reproduce real-world practice, in order to make more precise recommendations on how to incorporate this biomarker in the clinic, and we believe these results will be interesting to our community.
Please cite this article as: Golpe R, et al. Recuento de eosinófilos en sangre y eventos centrados en el paciente con enfermedad pulmonar obstructiva crónica en práctica asistencial real. Arch Bronconeumol. 2020;56:129–130.