Malignant pleural effusion (MPE) is a common manifestation of advanced cancer and is associated with breathlessness requiring therapeutic pleural interventions.1 Management is primarily palliative aiming at symptomatic relief and improving quality of life (QOL).2 Recent data demonstrate that patients with MPE have reduced physical activity (PA) levels leading to sedentary lifestyle.3
Accumulating evidence in different cancer populations suggests sedentary behaviour has a negative impact on physical and mental well-being and overall health-related QOL.4,5 A recent meta-analysis of 71 cohort studies found that maintaining PA was associated with lower mortality risk in cancer survivors, but cause and effect are difficult to disentangle.6
Assessment of PA has traditionally been conducted with the use of questionnaires, which are subjective and suitable for a long-term view of individual activity level.7 Activity monitors are used to more precisely map individual activity levels by either recording body movements in different planes over time, or calculating energy expenditure.7 Metabolic equivalent of tasks (MET) is a measure of an individual's functional capacity or aerobic power and is defined as the amount of oxygen consumed while sitting at rest.8 Sedentary behaviour is associated with MET<1.54 while activities such as gardening or climbing the stairs have a metabolic equivalent of 4 or more.8 The Sensewear® Armband (SWA) (BodyMedia, Pittsburgh, Pennsylvania, USA) has been validated as a sensitive device for evaluation of energy expenditure (and by extension PA) in patients with respiratory disease.9
It is not yet established whether therapeutic pleural interventions positively affect PA in MPE patients. This study examined changes in activity levels following therapeutic pleural interventions. Given the paucity of current evidence in this area, this pilot study assessed the feasibility of data collection and early initial signals in MPE patients post intervention.
ASPIRE was a prospective observational study of patients with MPE undergoing therapeutic pleural procedures and was approved by the South Central Research Ethics Committee in the UK(REF 18/SC/0011). No formal sample size calculation was made given the exploratory nature of the study. We aimed to recruit twenty patients from the Oxford Pleural Unit, to demonstrate feasibility of data collection. Adult patients with symptomatic MPE requiring drainage were included. Exclusion criteria were the presence of disabling neurological or musculoskeletal conditions (which would impact PA measurement), life expectancy<1 month or if the World Health Organisation Performance Status (PS) was>3 (essentially bedbound).
Baseline demographic data and breathlessness using an MPE validated score (100-mm visual analogue scale (VAS)) was measured immediately before and one hour to seven days post pleural intervention. PA levels were measured pre and post intervention; participants were asked to wear the SWA around the non-dominant upper arm during the awake period for a minimum of four days and up to ten days prior to pleural intervention. Following the therapeutic pleural procedure, patients were given the SWA to wear for a further seven days. Data retrieved from SWA for the two study periods included: total energy expenditure (TEE), step count, average metabolic equivalent of task (MET) and waking time spent in non-sedentary activity (≥1.5 MET). The average daily value for these parameters in the pre- and post-intervention was calculated. The primary endpoint for the study was change in PA level peri-intervention and the secondary endpoint was examining the relationship between change in PA and breathlessness.
Qualitative variables were expressed as frequencies and percentages while quantitative variables were expressed as medians and interquartile ranges (IQR). The Wilcoxon and Mann-Whitney tests were used to compare continuous variables as appropriate. Spearman correlation was used to examine the relation between symptoms and activity variables and volume of drained effusion.
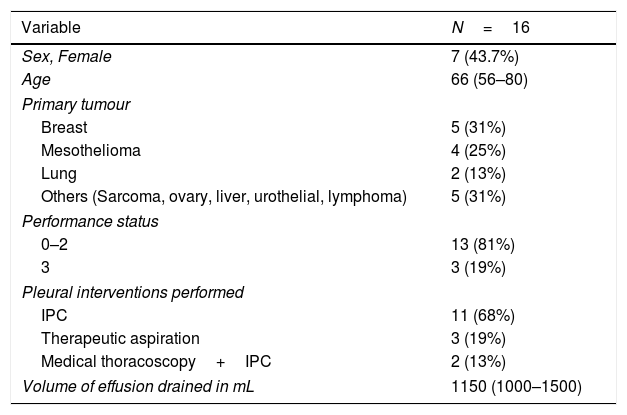
Twenty patients were recruited; four patients did not complete the post intervention assessments as stipulated in the protocol and hence 16 patients were included in the analysis. Baseline demographics and type of pleural procedure are presented in Table 1.
Characteristics of the included patients (n=16) and the pleural procedures performed. Data expressed as median (IQR) or frequency (percent).
| Variable | N=16 |
|---|---|
| Sex, Female | 7 (43.7%) |
| Age | 66 (56–80) |
| Primary tumour | |
| Breast | 5 (31%) |
| Mesothelioma | 4 (25%) |
| Lung | 2 (13%) |
| Others (Sarcoma, ovary, liver, urothelial, lymphoma) | 5 (31%) |
| Performance status | |
| 0–2 | 13 (81%) |
| 3 | 3 (19%) |
| Pleural interventions performed | |
| IPC | 11 (68%) |
| Therapeutic aspiration | 3 (19%) |
| Medical thoracoscopy+IPC | 2 (13%) |
| Volume of effusion drained in mL | 1150 (1000–1500) |
IQR: interquartile range; IPC: indwelling pleural catheter.
The median dyspnoea VAS score pre-intervention was 44 (21.8–77.3) mm, and post procedure was 31 (17.5–44) mm (Z −2.0, p=0.044) (supplementary figure). Nine patients (56.3%) achieved at least 19mm improvement in the VAS score which is the minimal clinically important difference (MCID).10
The SWA data for the pre and post-intervention periods is summarized in the supplementary table. The median TEE/day reduced from 7558 to 7039 (Z −1.7, p=0.09). The percent of waking time spent in non-sedentary activity/day reduced from 14.3 to 13.1% (Z −1.5, p=0.13) (supplementary figure). The median daily step count reduced from 785 to 245 steps (Z −3.10, p=0.002). There was no significant difference in the percent change in time spent in non-sedentary behaviour in those who achieved or did not achieve the MCID for breathlessness score (Z 1.16, p=0.25). A positive correlation was seen between the volume of pleural fluid drained and decrease in VAS score (R 0.55, p=0.044), while no significant correlation was seen between the change in average TEE/day and volume of drainage (R −0.14, p=0.63).
To our knowledge, this is the first study to examine prospectively the variation in PA levels pre- and post-therapeutic pleural procedures in patients with MPE, and provides evidence that it is feasible to study this parameter in such patients with acceptable adherence to the study intervention (wearing the activity armband) around the time of the pleural intervention.
The data from this study confirms the generally low levels of PA in this patient cohort, and suggests a dissociation between improvement in breathlessness following pleural drainage and change in PA. Fifty six percent (n=9) of our patients experienced a clinically significant improvement in breathlessness, and yet, even in this subgroup, the PA did not increase. We hypothesize that such patients are breathless even at rest, and thus the significant symptom relief gained post aspiration is largely experienced at rest and does not lead to increased PA. This has potentially important implications for future studies of physical activity in MPE patients, suggesting that measures of PA do not reflect the patient experience of improved breathlessness. However, we recognise that the small size of the sample might have caused any signal of changes in PA post intervention to be obscured. Additionally, it is recognised that in patients with MPE dyspnoea can have other causes besides the effusion such as parenchymal lung infiltration or lymphangitis in lung cancer or encasement of the ipsilateral lung by aggressive pleural malignancy particularly in mesothelioma.
One of the unexpected findings is that the PS for 81% of the patients was 2 or less, indicating that they spend<50% sitting or lying down during the day. However, the median time per day spent in non-sedentary activities measured objectively was only 14.3%. This highlights the discrepancy between clinician-ascribed PS and actual activity which has been associated with increased risk of mortality.11 The generally low functional reserve in patients newly diagnosed with cancer, and the challenge of carefully assessing this during brief clinical consultations, represent the underpinnings for prehabilitation, a set of interventions addressing physical and psychological issues in cancer patients before starting treatment with the aim of improving outcomes.12 The findings from our study support a potential role for rehabilitation/physiotherapy for patients with MPE.
This study is limited by the small number of participants and the high percentage of incomplete study assessments (20%). Some patients experience tenderness at the site of the procedure for 24–48h which can cause them to avoid mobilizing. This is a confounding factor that is challenging to account for and design of future studies examining PA in this population should take this into consideration when deciding on timings of measuring PA and administering symptoms questionnaires post interventions.
In conclusion, measuring PA in patients with MPE is feasible with good compliance and tolerability. The findings of this study suggest that this patient population experience poor levels of PA pre and post therapeutic interventions. Results from a currently recruiting large observational study on PA in MPE patients are awaited to shed more light on the subject (NCT03482570). Assessment of further interventions (for example physiotherapy) to address this issue should be tackled in future studies, and measurement of PA via our methodology should be feasible in such studies.
Authors’ contributionMH and NMR conceived the study and wrote the protocol. All authors recruited participants and collected data as part of the study. MH and RB performed the statistics and drafted the first manuscript. NMR critically revised the manuscript. All authors reviewed and approved the final manuscript. MH and NMR act as guarantors for the overall content of the manuscript.
Conflict of interestNone to declare.
DisclosureMH and OCA received fellowships from the European Respiratory Society to train at the Oxford Pleural Unit. Part of the data in the manuscript was presented in abstract form in the European Respiratory Society virtual congress in September 2020.
The authors would like to thank Melissa Dobson and Emma Hedley from the Oxford Respiratory Trials Unit for their help with the study protocol formulation and registration.