Telomeres shorten progressively during lifetime, and this has been proposed as one of the primary molecular causes of aging.1 Its deficiency produces multiple telomeric syndromes and age-related diseases, including pulmonary fibrosis.2 Likewise, the incidence of severe or fatal COVID-19 increases with age, suggesting that age-related molecular pathways contribute to the severity of this disease.3 Multiple studies have shown that patients with COVID-19 present fibrotic pulmonary sequelae, which in certain cases can persist in the long term.4 In a previous study by our group, we already showed that markers of fibrogenesis, specifically periostin, are higher in COVID-19 patients with fibrotic sequelae than in those without this complication.5 This implies that the lung fibrosis mechanism in COVID-19 is in some way similar to other fibrosing diseases such as idiopathic pulmonary fibrosis (IPF). Telomere shortening has also been linked to fibrotic diseases,6,7 the most prevalent and studied of which is IPF,8 and several studies are also linking telomere shortening to other types of nosological entities with a role in lung fibrosis development.9,10
Various authors have measured telomere length in peripheral blood cells of individuals with COVID-19, finding lower percentiles in length and a faster rate of telomere attrition in all age ranges of patients who developed more severe COVID-19 pathologies.11,12 On the other hand, it appears that there may also be a relationship between post COVID-19 sequelae and telomeres. In one study the authors correlated the appearance of post-COVID-19 syndrome (fatigue, dyspnea, memory loss, etc.) with telomeric shortening.13 Besides that, telomere length has been measured in ATII cells in post-COVID-19 patients, finding shorter telomeres and concomitant fibrotic lung parenchyma remodeling, compared to controls, suggesting a link between them.14
In light of this, in our study we aimed to analyze the evolution of telomere length in patients admitted for COVID-19 and its link to radiological fibrotic sequelae 12 months after infection.
We analyzed data from 19 patients admitted for bilateral COVID-19 pneumonia from the COVID-FIBROTIC cohort study.4 Mean age was 62.4 years and 84.2% were men. At one year after discharge we measured pulmonary function tests, finding that 5% had a forced vital capacity (FVC)<80% and 50% had a diffusion lung capacity for CO (DLCO)<80%. We also found that 63.2% of patients had fibrotic changes on HRCT.
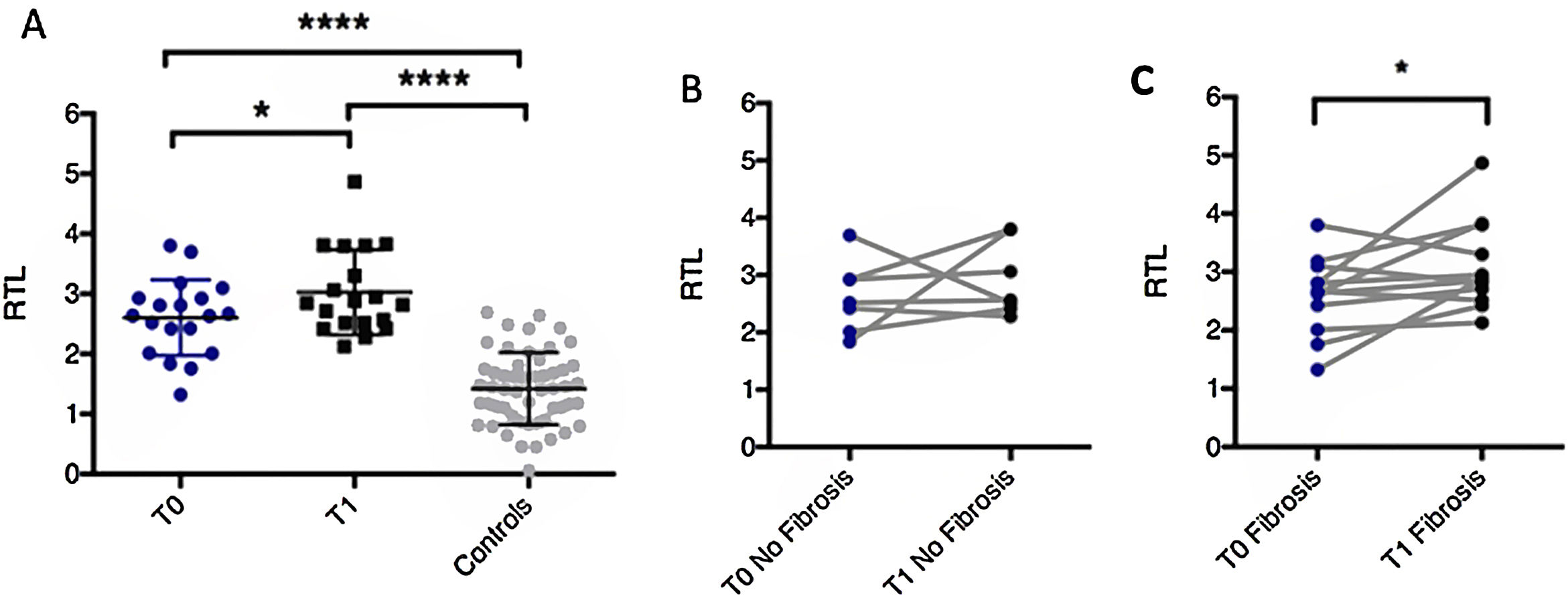
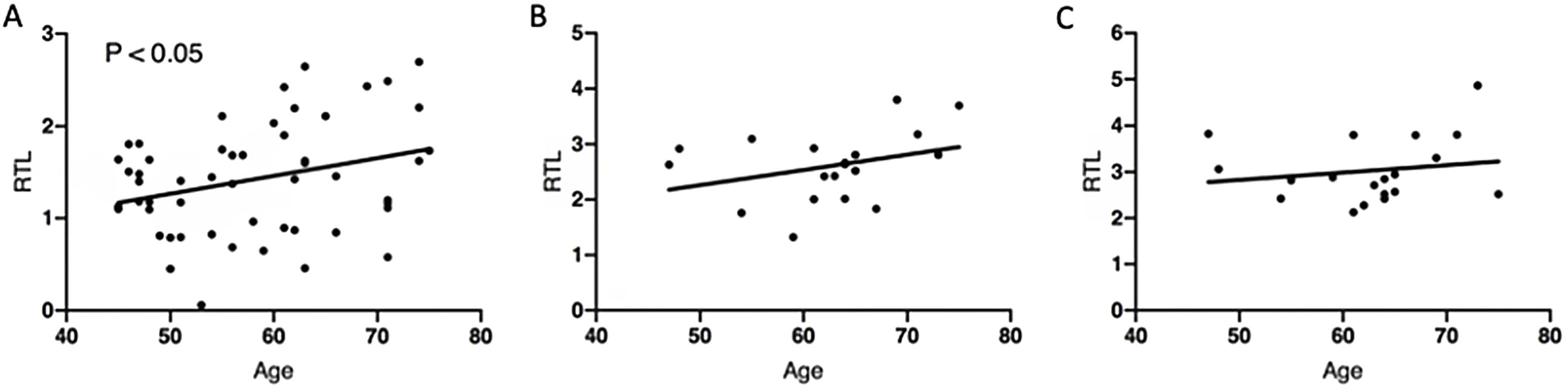
We measured relative telomere length in peripheral blood leukocytes from patients at two different times (evolution group): at admission (T0), and at one year after discharge (T1), observing significant telomere shortening (T0 mean=2.605±0.1453; T1 mean=3.028±0.1617; p-value=0.0383) (Fig. 1A). Comparing the evolution group with 60 human matched controls, we noticed a substantial difference in RTL (relative telomere length) values, confirming that SARS-CoV-2 infection decreased telomere length (control mean RTL=1.421±0.0825). Specifically, 94.74% of patients were found to have shorter telomeres compared to age- and sex-matched controls at one year after admission. The correlation between shortening and age that occurs physiologically was not observed in our group of patients (Fig. 2).
Evolution of telomere shortening over one year after severe COVID-19. (A) Representation of relative telomere length (RTL) of patients admitted for bilateral COVID-19 pneumonia at admission and 1 year after discharge compared to controls. (B) RTL changes in patients without fibrosis. (C) RTL changes in patients with fibrosis.
Correlation between age and relative telomere length (RTL). Pearson correlation analysis between RTL and age in controls (A), in patients at T0 (B) and in patients at T1 (C). Bivariate correlation was made using Pearson's coefficient (A: r=0.297; B: r=0.362; C: r=0.170). Age is expressed in years. Telomere length is expressed as RTL. T0: at admission; T1: one year after discharge.
Among our cohort of patients some showed shortened telomere length compared to the initial value as measured on admission while others did not, so we split patients in two groups according to this shortening for further comparison. No between-group differences were found in terms of demographic and clinical characteristics. However, patients without telomeric shortening showed an improvement in pulmonary function one year after discharge, compared to the tests performed at the first visit after discharge (at approximately two months), with statistical significance for the increase in DLCO (p-value=0.018).
We found that 63.2% of patients had fibrotic sequelae one year after admission. Patients were divided according to presence or absence of fibrotic changes showing that those with radiological signs of these changes had significant telomere shortening one year after leaving the hospital, whereas those not developing fibrotic changes had no significant telomeric shortening (Fig. 1B, C). We evaluated two well known risk factors related to increased fibrosis: age, and presence of the MUC5B polymorphism.15 In our patient series, mean age was the same in both groups, and no significant positive association was observed between age and fibrosis development. Likewise, single-nucleotide MUC5B polymorphisms (rs35705950) were tested and evaluated for associations with fibrotic sequelae in our patients. The mean frequency of the MUC5B rs35705950 T/G polymorphism in the COVID-19 group was 0.105, similar to the T-allele frequency of 0.118 in the European group.16 No differences were observed between patients with or without attrition in terms of invasive mechanical ventilation (IMV) use, which was more frequent in the group without shortening. This finding ruled out fibrotic changes as secondary to a possible complication of ventilation use. Consequently, both patient groups (with or without telomere attrition) are homogeneous groups and seemingly only COVID-19 could be responsible for this shortening, with no other external factors that could favor it. Therefore, in our cohort it seems that fibrosis is produced solely by SARS-CoV-2. Furthermore, in this pilot study, we demonstrate for the first time the relationship between peripheral blood leukocytes telomere shortening and long-term fibrotic changes in patients one year after COVID-19.
In conclusion, our data suggests that SARS COV2 causes peripheral blood leukocyte telomere attrition one year after infection, and this shortening is associated with fibrotic pulmonary sequelae. Our findings, when confirmed in larger studies, could have great repercussions, above all because of the large number of people affected worldwide, and because of the implications of telomeric shortening, which is described as one of the main molecular mechanisms of aging.17 It would also mean that people who have had severe COVID-19 disease with bilateral pneumonia would have premature cellular aging, with all the consequences that this entails. Telomere length studies could also play a crucial role in deciding the long-term follow-up of these patients. In addition, the telomeric attrition detected in ATII cells also exists in monocytes; several studies have shown that the measurement at the two sites is equivalent.18 Therefore, its measurement in peripheral blood is an easily monitored parameter in clinical practice.
Authors’ contributionsAll authors contributed to the literature search, study design, data interpretation, drafting manuscript and critical revision of the work. All authors had full access to all the data and contributed to article writing and editing and approved its submission. All study team members supported the implementation, sampling and data submission. All authors read and approved the final manuscript.
FundingThis work was supported by the Instituto de Salud Carlos III (COV20/01209); grant from the Valencian Society of Pneumology and COVID-19 funds from the Generalitat Valenciana (GVA).
Conflict of interestNone.