Although the medium- and long-term sequelae of survivor of acute respiratory distress syndrome (ARDS) of any cause have been documented, little is known about the way in which COVID-19-induced ARDS affects functional disability and exercise components. Our aims were to examine the medium-term disability in severe COVID-19-associated ARDS survivors, delineate pathophysiological changes contributing to their exercise intolerance, and explore its utility in predicting long-term functional impairment persistence.
MethodsWe studied 108 consecutive subjects with severe COVID-19 ARDS who remained alive 6 months after intensive care unit (ICU) discharge. Lung morphology was assessed with chest non-contrast CT scans and CT angiography. Functional evaluation included spirometry, plethysmography, muscle strength, and diffusion capacity, with assessment of gas exchange components through diffusing capacity of nitric oxide. Disability was assessed through an incremental exercise test, and measurements were repeated 12 and 24 months later in patients with functional impairments.
ResultsAt 6 months after ICU discharge, a notable dissociation between morphological and clinical-functional sequelae was identified. Moderate-severe disability was present in 47% of patients and these subjects had greater limitation of ventilatory mechanics and gas exchange, as well as greater symptomatic perception during exercise and a probable associated cardiac limitation. Female sex, hypothyroidism, reduced membrane diffusion component, lower functional residual capacity, and high-attenuation lung volume were independently associated with the presence of moderate-severe functional disability, which in turn was related to higher frequency and greater intensity of dyspnea and worse quality of life. Out of the 71 patients with reduced lung volumes or diffusion capacity at 6 months post-ICU discharge, only 19 maintained a restrictive disorder associated with gas exchange impairment at 24 months post-discharge. In these patients, 6-month values for diffusion membrane component, maximal oxygen uptake, ventilatory equivalent for CO2, and dead space to tidal volume ratio were identified as independent risk factors for persistence of long-term functional sequelae.
ConclusionsLess than half of survivors of COVID-19 ARDS have moderate-severe disability in the medium term, identifying several risk factors. In turn, diffusion membrane component and exercise tolerance at 6-month ICU discharge are independently associated with the persistence of long-term functional sequelae.
Acute respiratory distress syndrome (ARDS) significantly contributes to COVID-19 mortality, leading to respiratory failure and multi-organ dysfunction.1,2 Despite improved understanding of COVID-19 ARDS pathophysiology,3 survivors still worry about potential long-term respiratory issues, yet information is limited. Physiological differences, particularly in oxygenation, pulmonary mechanics, and treatment response, exist between COVID-19 ARDS and other ARDS variants.4 Therefore, prior insights into non-COVID-19 ARDS long-term outcomes5 may not be directly applicable to COVID-19 patients. Similarly, data on sequelae from severe COVID-19 pneumonia cannot be directly extrapolated to ARDS, since these represent distinct spectrums of COVID-19, characterized by notable differences in lung damage and pathogenic mechanisms.6
To date, disability assessment in survivors of COVID-19-related ARDS has predominantly relied on the six-minute walk test7–11 in studies characterized by small sample sizes, brief follow-up periods, and varying severity levels. Consequently, there is limited information available on the impact of COVID-19 ARDS on the diverse pathophysiological pathways implicated in exercise intolerance, necessitating a more comprehensive evaluation using a cardiopulmonary exercise test.12 Moreover, there is insufficient data on the determinants of medium-term disability and its long-term influence on residual lung function.
Hence, our objective was to investigate the extent of medium-term disability among survivors of severe COVID-19-associated ARDS, while delineating the pathophysiological changes contributing to their exercise intolerance and exploring its usefulness in estimating the risk of persistence of long-term functional impairment. Through a comprehensive assessment that incorporates clinical, radiological, and functional parameters, we also aimed to identify the primary risk factors associated with these changes and analyze their long-term impact on patients’ pulmonary function.
MethodsStudy design and patientsThis is an ambispective observational study of patients with severe COVID-19 ADRS who were discharged from our hospital between February 2020 and January 2021.
Patients were eligible for enrolment if they were 18 years of age or older and had survived severe COVID-19 ARDS (defined using the Berlin criteria), requiring invasive mechanical ventilation for at least 7 days, with SARS-CoV-2 infection confirmed at that time by positive reverse-transcriptase polymerase chain reaction on either a nasal swab or tracheal aspirate. Participants provided their written consent, and the study was approved by the institutional Ethics Committee (PI-4189). A more detailed description of the selection criteria is provided on the Online Data Supplement. We reported results in accordance with the STROBE guidelines.
MeasurementsWe recorded data for demographic variables, clinical characteristics, patient management, and complications during their ICU stay. Six months after ICU discharge, anthropometric parameters, smoking status, comorbidities and current treatment were registered. Respiratory symptoms were assessed using the European Community for Coal and Steel Questionnaire and the modified Medical Research Council dyspnea scale, whereas health-related quality of life was evaluated with the Spanish version of the Medical Outcomes Study 12-Item Short-Form Health Survey (SF-12). Further details on the clinical evaluation are presented in the Additional file (https://doi.org/10.1016/j.arbres.2024.05.021).
Lung morphology was assessed with a non-contrast chest CT scan and a CT angiography (CTA) scan with iodine mapping. CT images were evaluated by 2 chest radiologists with 10 years of experience and blinded to clinical data using systematized terminology to describe CT abnormalities and their extension.13 Moreover, a quantitative analysis of lung attenuation was performed using automated software. Additional details of image studies are provided in the Additional file.
Spirometry, plethysmography and diffusing capacity of the lungs for carbon monoxide (DLCO) measurements were performed using MasterScreen (Viasys, CareFusion, Germany) in accordance with current standardization.14–16 Global Lung Initiative equations were used as reference values, and tests were interpreted according to European Respiratory Society (ERS)/American Thoracic Society (ATS) strategies.17 Lung diffusing capacity for nitric oxide (DLNO) was also measured following ERS recommendations.18 The membrane component of diffusing capacity (Dm) and the pulmonary capillary blood volume (Vc) were calculated, and reference equations proposed by Zavorsky et al. were used.18 Respiratory muscle strength was assessed by maximal inspiratory mouth pressure, hand-grip strength was measured with a dynamometer, and limb muscle weakness was defined as a value <10th percentile of reference value.19 Patients with evidence of restrictive disorder or impaired gas exchange were reevaluated 12 months after ICU discharge and again 24 months later when these alterations persisted. A more detailed description of all lung tests is provided in the Additional file.
An incremental cycle exercise test was conducted on a cycle ergometer (Vintus CPX, Carefusion) following standards of the ATS/American College of Chest Physicians (ACCP) statement20 and using the prediction equations of Puente-Maestú21 (further details in the Additional file). Changes in operational lung volumes were evaluated to identify dynamic hyperinflation, and functional capacity was graded according to peak oxygen uptake (normal: >20, mild: 16-20, moderate: 10-16, and severe: <10mL/kg/min).22
Statistical analysisThe sample size was estimated under the arbitrary assumption of a 20% prevalence of moderate-to-severe disability among survivors of COVID-19-related ARDS by extrapolation of the disability degree reported in survivors of severe COVID-19 pneumonia.23 To determine its frequency in an unknown population with a precision of 5% and a confidence level of 95%, 107 patients were required.
Categorical variables are presented as numbers with percentages, and continuous variables as mean with standard deviation (SD) or median (interquartile range), according to their distribution. Comparisons between subgroups were performed using the Student's t-test, ANOVA, Mann-Whitney U-test, Kruskal-Wallis or chi-squared test. Comparisons of lung function values 6, 12 and 24 months after ICU discharge were performed using general linear models of repeated measures. The relationship between variables was assessed with the Pearson correlation analysis. Multiple logistic regression analysis was used to study factors associated with functional or morphologic impairment and disability. Only variables with a level of significance <0.05 in the bivariate analysis were included in the stepwise multiple regression models, and odds ratios were calculated. Data were analyzed with the R (Venn-Euler package) and SPSS 25.0 software (IBM, Chicago, IL, USA), considering a P-value <0.05 statistically significant for all tests.
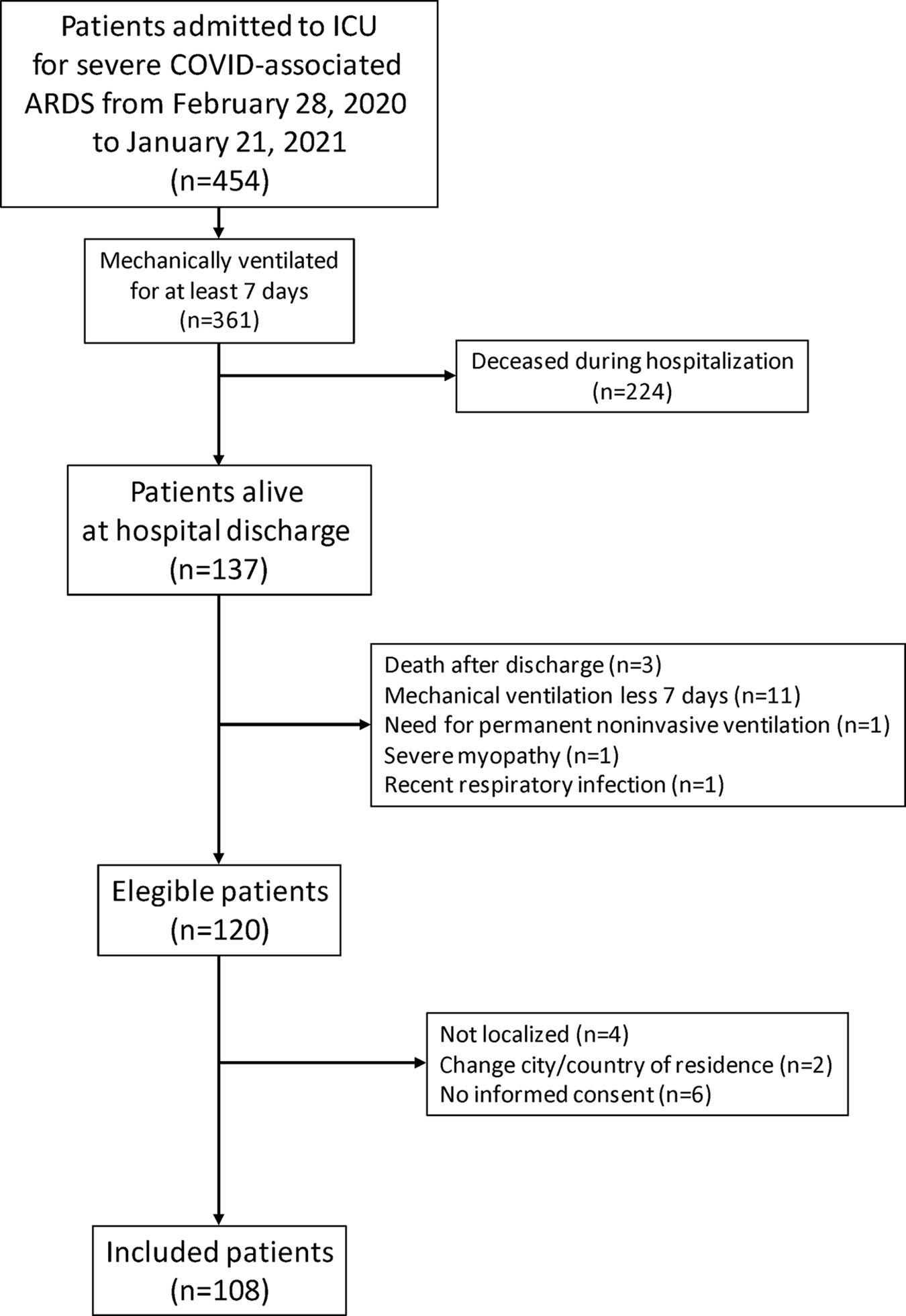
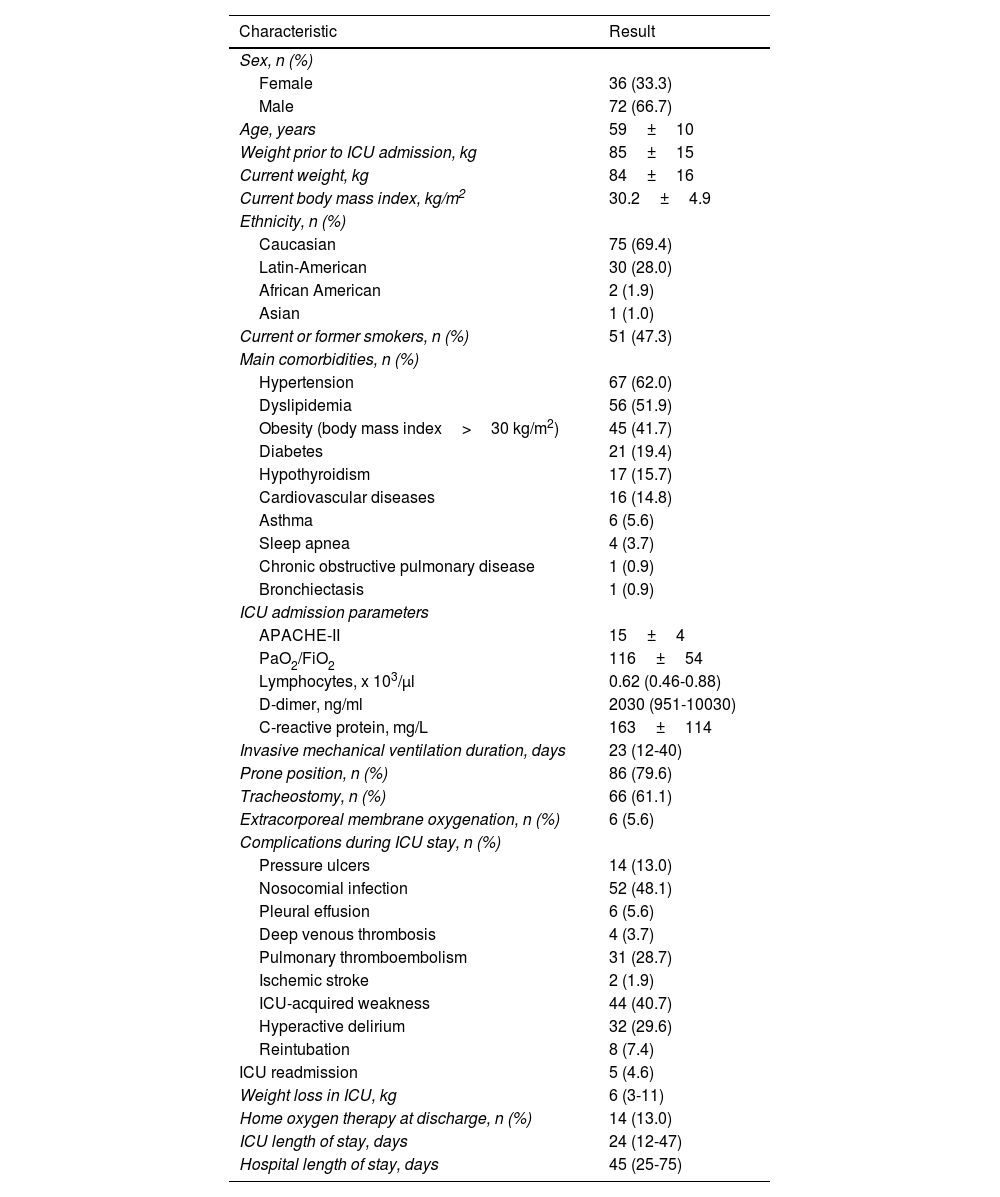
ResultsFigure 1 shows the patient recruitment flowchart, and Table 1, along with Tables S1 and S2 in the Additional file, summarize the main characteristics of the 108 patients finally selected.
General characteristics of the study subjects.
| Characteristic | Result |
|---|---|
| Sex, n (%) | |
| Female | 36 (33.3) |
| Male | 72 (66.7) |
| Age, years | 59±10 |
| Weight prior to ICU admission, kg | 85±15 |
| Current weight, kg | 84±16 |
| Current body mass index, kg/m2 | 30.2±4.9 |
| Ethnicity, n (%) | |
| Caucasian | 75 (69.4) |
| Latin-American | 30 (28.0) |
| African American | 2 (1.9) |
| Asian | 1 (1.0) |
| Current or former smokers, n (%) | 51 (47.3) |
| Main comorbidities, n (%) | |
| Hypertension | 67 (62.0) |
| Dyslipidemia | 56 (51.9) |
| Obesity (body mass index>30 kg/m2) | 45 (41.7) |
| Diabetes | 21 (19.4) |
| Hypothyroidism | 17 (15.7) |
| Cardiovascular diseases | 16 (14.8) |
| Asthma | 6 (5.6) |
| Sleep apnea | 4 (3.7) |
| Chronic obstructive pulmonary disease | 1 (0.9) |
| Bronchiectasis | 1 (0.9) |
| ICU admission parameters | |
| APACHE-II | 15±4 |
| PaO2/FiO2 | 116±54 |
| Lymphocytes, x 103/μl | 0.62 (0.46-0.88) |
| D-dimer, ng/ml | 2030 (951-10030) |
| C-reactive protein, mg/L | 163±114 |
| Invasive mechanical ventilation duration, days | 23 (12-40) |
| Prone position, n (%) | 86 (79.6) |
| Tracheostomy, n (%) | 66 (61.1) |
| Extracorporeal membrane oxygenation, n (%) | 6 (5.6) |
| Complications during ICU stay, n (%) | |
| Pressure ulcers | 14 (13.0) |
| Nosocomial infection | 52 (48.1) |
| Pleural effusion | 6 (5.6) |
| Deep venous thrombosis | 4 (3.7) |
| Pulmonary thromboembolism | 31 (28.7) |
| Ischemic stroke | 2 (1.9) |
| ICU-acquired weakness | 44 (40.7) |
| Hyperactive delirium | 32 (29.6) |
| Reintubation | 8 (7.4) |
| ICU readmission | 5 (4.6) |
| Weight loss in ICU, kg | 6 (3-11) |
| Home oxygen therapy at discharge, n (%) | 14 (13.0) |
| ICU length of stay, days | 24 (12-47) |
| Hospital length of stay, days | 45 (25-75) |
Values are mean±standard deviation, median (interquartile range) or number (percentage) according to their type and distribution.
Abbreviations: ICU: Intensive Care Unit; APACHE: acute physiology, age, and chronic health evaluation; PaO2/FiO2: ratio of arterial oxygen partial pressure to fractional inspired oxygen.
Frequency and characteristics of respiratory symptoms and health-related quality of life are presented in Fig. S1 and Table S3. Among the 100 patients with available CT scans six months post-ICU discharge, only 10 showed complete resolution of lung abnormalities (Table S4). Specifically, 10 patients exhibited unequivocal signs of established fibrosis, 73 presented fibrotic-like abnormalities and 7 displayed non-fibrotic abnormalities. Additionally, patchy perfusion defects were identified in 25 out of 70 patients with interpretable iodine maps (35.7%). Persistent established fibrosis or fibrotic-type abnormalities were associated with the number of previous comorbidities and nosocomial infection during ICU stay (Table S5).
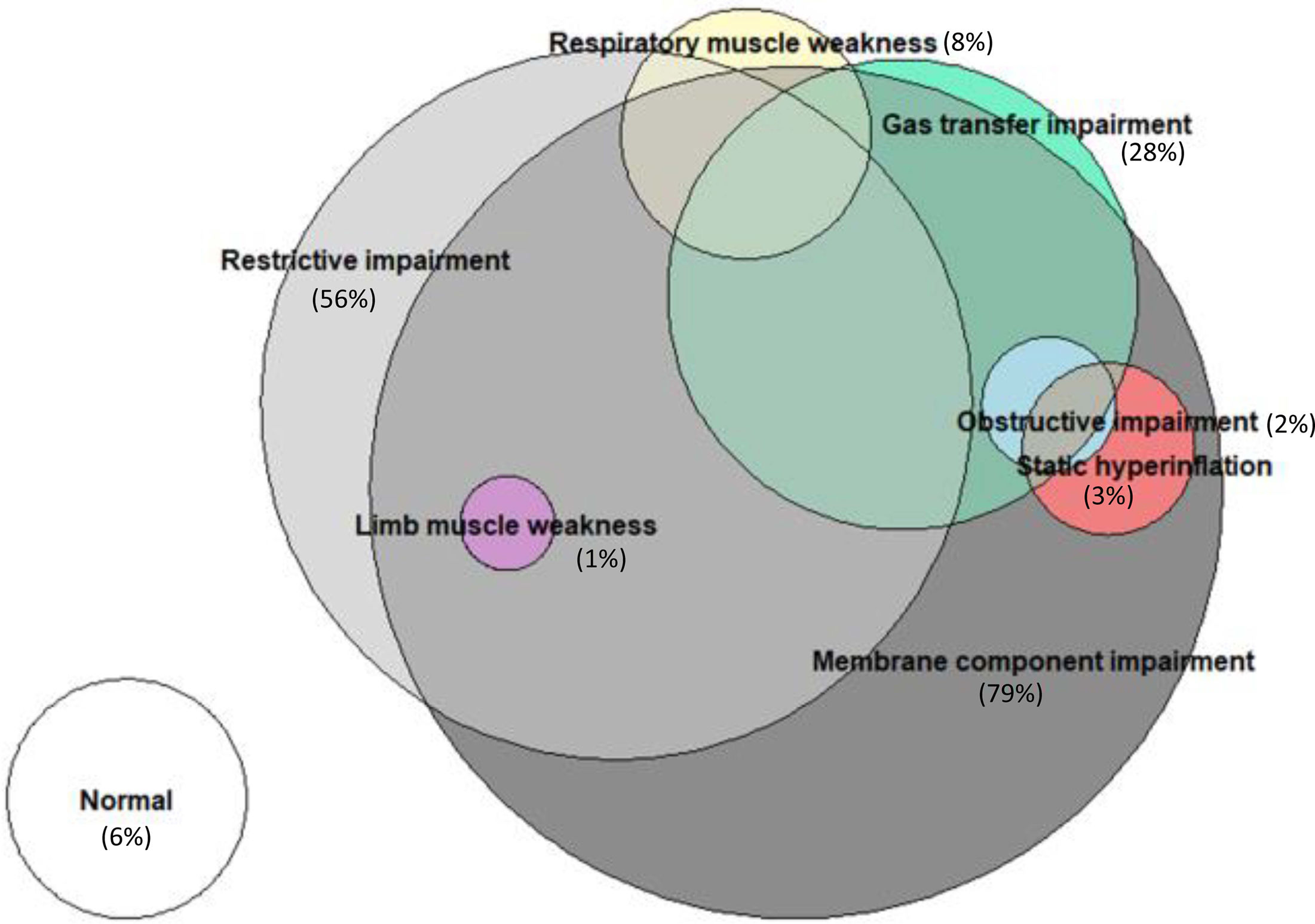
Regarding conventional lung function (Table S6), restrictive impairment was the most prevalent functional disorder (57%), with impaired gas transfer observed in 30 patients (28%) (Fig. S2, Table S7). A detailed examination of gas exchange components through DLNO (Table S8) revealed membrane component involvement in almost all patients with low DLCO, which also remained reduced in a substantial percentage of patients with normal DLCO (Fig. 2). Lymphopenia at ICU admission emerged as the only independent risk factor associated with restrictive impairment, while the absence of obesity, hypothyroidism and days of invasive mechanical ventilation were independently associated with the presence of gas transfer impairment (Table S9). Additionally, male sex, age and the number of previous comorbidities were independent determinants of membrane component impairment (Table S9).
Gas exchange impairment was associated with the presence of dyspnea (OR 2.53, 95%CI 1.07-6.01, P=0.035) or any respiratory symptom (OR 5.50, 95%CI 1.21-25.07, P=0.028), with dyspnea severity higher in patients with restrictive disorder, gas exchange impairment, or low membrane component involvement (Fig. S3). Furthermore, a CT pattern with unequivocal signs of established fibrosis was associated with restrictive impairment, whereas an increase in high attenuation volume was associated with both restrictive disorder and gas transfer impairment (Table S10).
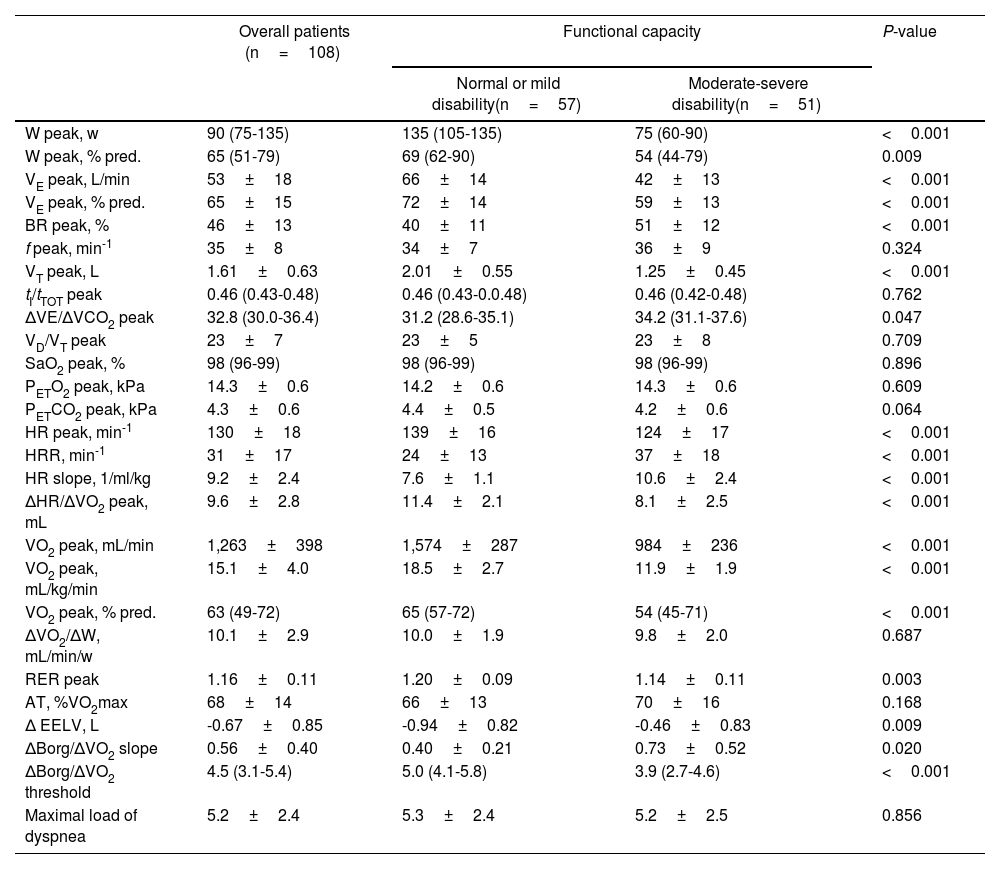
Functional disability at six months after ICU dischargeExercise testing revealed normal functional capacity in 12 patients (11.1%), mild disability in 45 (41.7%), moderate disability in 42 (38.9%) and severe disability in 9 (8.3%). Table 2 presents the exercise response parameters of all patients and compares those with moderate-severe disability to the remaining patients. Patients with moderate-severe disability exhibited lower minute ventilation due to limited tidal volume expansion and lower end-expiratory lung volume decrease, alongside higher peak carbon dioxide ventilatory equivalent, suggesting the contribution of both ventilatory mechanics and gas exchange in their functional limitation (Table 2). This was accompanied by increased symptomatic perception during exercise, as well as potential cardiac limitation suggested by the lower peak oxygen pulse and higher slope of heart rate response to exercise.
Exercise parameters six months after ICU discharge in overall patients and according to their functional capacity.
| Overall patients (n=108) | Functional capacity | P-value | ||
|---|---|---|---|---|
| Normal or mild disability(n=57) | Moderate-severe disability(n=51) | |||
| W peak, w | 90 (75-135) | 135 (105-135) | 75 (60-90) | <0.001 |
| W peak, % pred. | 65 (51-79) | 69 (62-90) | 54 (44-79) | 0.009 |
| VE peak, L/min | 53±18 | 66±14 | 42±13 | <0.001 |
| VE peak, % pred. | 65±15 | 72±14 | 59±13 | <0.001 |
| BR peak, % | 46±13 | 40±11 | 51±12 | <0.001 |
| f peak, min-1 | 35±8 | 34±7 | 36±9 | 0.324 |
| VT peak, L | 1.61±0.63 | 2.01±0.55 | 1.25±0.45 | <0.001 |
| tI/tTOT peak | 0.46 (0.43-0.48) | 0.46 (0.43-0.0.48) | 0.46 (0.42-0.48) | 0.762 |
| ΔVE/ΔVCO2 peak | 32.8 (30.0-36.4) | 31.2 (28.6-35.1) | 34.2 (31.1-37.6) | 0.047 |
| VD/VT peak | 23±7 | 23±5 | 23±8 | 0.709 |
| SaO2 peak, % | 98 (96-99) | 98 (96-99) | 98 (96-99) | 0.896 |
| PETO2 peak, kPa | 14.3±0.6 | 14.2±0.6 | 14.3±0.6 | 0.609 |
| PETCO2 peak, kPa | 4.3±0.6 | 4.4±0.5 | 4.2±0.6 | 0.064 |
| HR peak, min-1 | 130±18 | 139±16 | 124±17 | <0.001 |
| HRR, min-1 | 31±17 | 24±13 | 37±18 | <0.001 |
| HR slope, 1/ml/kg | 9.2±2.4 | 7.6±1.1 | 10.6±2.4 | <0.001 |
| ΔHR/ΔVO2 peak, mL | 9.6±2.8 | 11.4±2.1 | 8.1±2.5 | <0.001 |
| VO2 peak, mL/min | 1,263±398 | 1,574±287 | 984±236 | <0.001 |
| VO2 peak, mL/kg/min | 15.1±4.0 | 18.5±2.7 | 11.9±1.9 | <0.001 |
| VO2 peak, % pred. | 63 (49-72) | 65 (57-72) | 54 (45-71) | <0.001 |
| ΔVO2/ΔW, mL/min/w | 10.1±2.9 | 10.0±1.9 | 9.8±2.0 | 0.687 |
| RER peak | 1.16±0.11 | 1.20±0.09 | 1.14±0.11 | 0.003 |
| AT, %VO2max | 68±14 | 66±13 | 70±16 | 0.168 |
| Δ EELV, L | -0.67±0.85 | -0.94±0.82 | -0.46±0.83 | 0.009 |
| ΔBorg/ΔVO2 slope | 0.56±0.40 | 0.40±0.21 | 0.73±0.52 | 0.020 |
| ΔBorg/ΔVO2 threshold | 4.5 (3.1-5.4) | 5.0 (4.1-5.8) | 3.9 (2.7-4.6) | <0.001 |
| Maximal load of dyspnea | 5.2±2.4 | 5.3±2.4 | 5.2±2.5 | 0.856 |
Values are mean±standard deviation or median (interquartile range) according to their distribution.
Abbreviations: W: work intensity; VE: minute ventilation; BR: breathing reserve; f: respiratory frequency; VT: tidal volume; tI: inspiratory time; tTOT: total respiratory time; ΔVE/ΔVCO2: ventilatory equivalent for carbon dioxide; VD/VT: ratio of physiologic dead space to tidal volume; SaO2: oxygen saturation; PETO2: end-tidal pressure of oxygen; PETCO2: end-tidal pressure of carbon dioxide; HR: heart rate; HRR: heart rate reserve; ΔHR/ΔVO2: oxygen pulse; VO2: oxygen uptake; RER: respiratory exchange ratio; AT: anaerobic threshold; ΔEELV: change from baseline in end-expiratory lung volume.
The presence of moderate-severe disability was associated with a higher frequency of respiratory symptoms and dyspnea, as well as greater intensity of dyspnea and worse quality of life in both physical and mental components (Table S11).
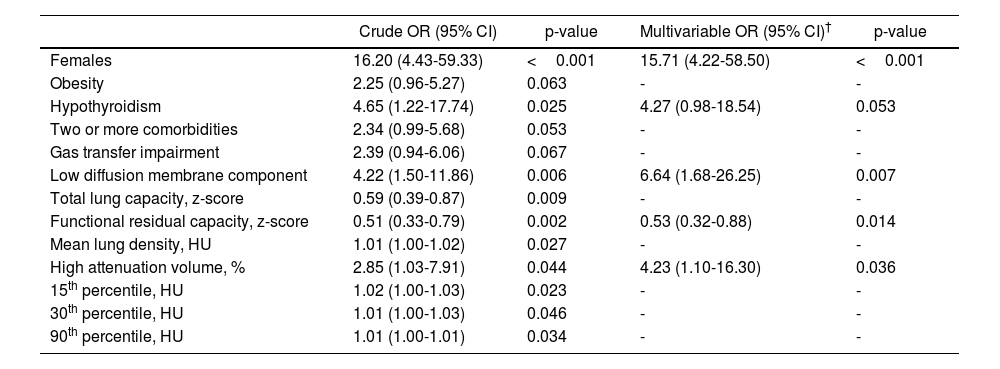
Table 3 shows the clinical, morphological and functional factors associated with the presence of moderate-severe disability. In a multivariate model, female sex, hypothyroidism, reduced membrane component of diffusion, lower functional residual capacity and the volume of lung parenchyma areas with high attenuation density were identified as independent risk factors for moderate-severe disability.
Risk factors for the presence of moderate-severe disability at 6 months of ICU discharge.
| Crude OR (95% CI) | p-value | Multivariable OR (95% CI)† | p-value | |
|---|---|---|---|---|
| Females | 16.20 (4.43-59.33) | <0.001 | 15.71 (4.22-58.50) | <0.001 |
| Obesity | 2.25 (0.96-5.27) | 0.063 | - | - |
| Hypothyroidism | 4.65 (1.22-17.74) | 0.025 | 4.27 (0.98-18.54) | 0.053 |
| Two or more comorbidities | 2.34 (0.99-5.68) | 0.053 | - | - |
| Gas transfer impairment | 2.39 (0.94-6.06) | 0.067 | - | - |
| Low diffusion membrane component | 4.22 (1.50-11.86) | 0.006 | 6.64 (1.68-26.25) | 0.007 |
| Total lung capacity, z-score | 0.59 (0.39-0.87) | 0.009 | - | - |
| Functional residual capacity, z-score | 0.51 (0.33-0.79) | 0.002 | 0.53 (0.32-0.88) | 0.014 |
| Mean lung density, HU | 1.01 (1.00-1.02) | 0.027 | - | - |
| High attenuation volume, % | 2.85 (1.03-7.91) | 0.044 | 4.23 (1.10-16.30) | 0.036 |
| 15th percentile, HU | 1.02 (1.00-1.03) | 0.023 | - | - |
| 30th percentile, HU | 1.01 (1.00-1.03) | 0.046 | - | - |
| 90th percentile, HU | 1.01 (1.00-1.01) | 0.034 | - | - |
Only anthropometric, clinical characteristics and ICU parameters significantly associated with each lung function impairment pattern are provided.
Abbreviations: OR: odds ratio; CI: confidence interval; HU: Hounsfield units.
At 24 months after ICU discharge, only 47 patients (44%) exhibited persistent restrictive disorder, also presenting impaired gas exchange 22 of them (23%). Fig. S4 shows the long-term evolution of total lung capacity (TLC) and DLCO in patients with reduced values 6 months after ICU discharge.
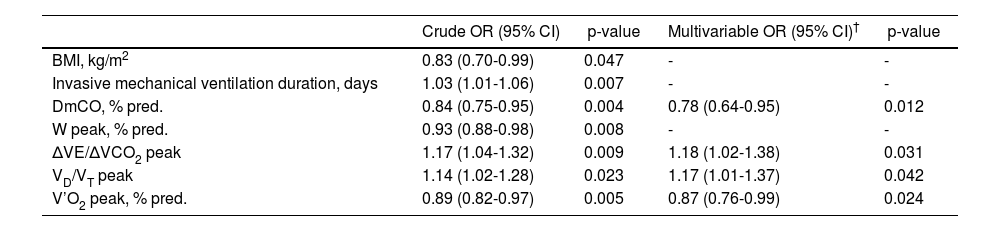
Among patients with restrictive impairment or reduced gas exchange at six months post-ICU discharge, the diffusion membrane component as well as the peak values of oxygen uptake, ratio of physiologic dead space to tidal volume and ventilatory equivalent for carbon dioxide and oxygen uptake were identified as independent risk factors for the long-term persistence of both functional disorders (Table 4), suggestive of established lung fibrosis.
Risk factors associated with long-term persistence of a restrictive disorder with impaired gas exchange among patients with reduction of either of both at six months after ICU discharge.
| Crude OR (95% CI) | p-value | Multivariable OR (95% CI)† | p-value | |
|---|---|---|---|---|
| BMI, kg/m2 | 0.83 (0.70-0.99) | 0.047 | - | - |
| Invasive mechanical ventilation duration, days | 1.03 (1.01-1.06) | 0.007 | - | - |
| DmCO, % pred. | 0.84 (0.75-0.95) | 0.004 | 0.78 (0.64-0.95) | 0.012 |
| W peak, % pred. | 0.93 (0.88-0.98) | 0.008 | - | - |
| ΔVE/ΔVCO2 peak | 1.17 (1.04-1.32) | 0.009 | 1.18 (1.02-1.38) | 0.031 |
| VD/VT peak | 1.14 (1.02-1.28) | 0.023 | 1.17 (1.01-1.37) | 0.042 |
| V’O2 peak, % pred. | 0.89 (0.82-0.97) | 0.005 | 0.87 (0.76-0.99) | 0.024 |
Only anthropometric, clinical characteristics and ICU parameters significantly associated with each lung function impairment pattern are provided.
Abbreviations: OR: odds ratio; CI: confidence interval; DmCO: diffusion membrane component; W: work intensity; VD/VT: ratio of physiologic dead space to tidal volume; ΔVE/ΔVCO2: ventilatory equivalent for carbon dioxide; VO2: oxygen uptake.
The main finding of our study is the demonstration that nearly half of the survivors of severe ARDS secondary to COVID-19 exhibit moderate to severe functional disability in the medium term. This impairment is more common in women, individuals with a history of hypothyroidism, and in the presence of concurrent reductions in residual functional capacity, increased lung parenchyma attenuation, and decreased diffusion membrane component. Additionally, both progressive cardiorespiratory exercise testing and analysis of the diffusion membrane component provide additional value in predicting long-term functional deterioration among those survivors who, at six months post-ICU discharge, exhibit reduced TLC or DLCO values. Thus, both the diffusion membrane component and exercise tolerance, along with parameters related to dead space and gas exchange at peak exercise, are independently associated with the persistence of a restrictive disorder associated with gas exchange impairment at 24 months post-discharge, suggestive of lung fibrosis development.
Considering the notable prevalence of post-intensive care syndrome observed in the months after discharge from COVID-19-associated ARDS,24 coupled with the documented progressive enhancement in exercise tolerance throughout the convalescent phase,5 we opted to conduct the initial assessment six months post-discharge. This timeframe aligns with previous investigations assessing exercise tolerance via the walking test among survivors of COVID-19-related ARDS,25,26 reflecting a consensus in the field regarding the optimal timing for such evaluations.
The incidence of restrictive impairment in our patients was slightly higher than in other COVID-19 ARDS series (where lung volumes weren’t assessed by plethysmography),27–29 or ARDS of other origin.30 This might be due to the severity of our cohort, since restrictive impairment is more common in COVID-19 patients admitted to the ICU requiring invasive ventilation compared to those managed with high-flow nasal oxygen or non-invasive ventilation.9
Surprisingly, only 28% of our patients showed signs of gas exchange impairment, a notably lower rate compared to survivors of severe COVID-19 pneumonia31 or other COVID-19 ARDS.32 Previous studies often defined gas exchange impairment based on varying DLCO percentages, rather than its lower limit of normal (LLN). When LLN criteria were applied, studies indicated a prevalence of gas exchange impairment in COVID-19 severe pneumonia patients ranging from 17% to 34%.33,34 Our findings clarify that the impaired gas exchange observed in these patients primarily stems from alveolar-capillary membrane damage rather than vascular issues. Intriguingly, recent data from non-critical patients recovering from severe COVID-19 pneumonia revealed a correlation between reduced DLCO at 3- and 12-months post-discharge and membrane factor involvement.34,35
We also explore the dissociation between morphological and clinical-functional sequalae. Whereas a restrictive or gas exchange impairment was related with higher dyspnea levels, no differences were observed between respiratory symptoms and the existence or not of parenchymal alterations. Furthermore, unequivocal signs of established fibrosis were only associated with restrictive impairment, not reduced DLCO. These findings partly agree with previous studies showing no association between imaging abnormalities and reduced lung function or symptoms in severe COVID-19 cases.28,31 This observation could be explained by the limited extent of the lesions, typically not exceeding 25% of the lung parenchyma, as seen in other studies.29 Hence, quantitatively assessing lung parenchymal attenuation might provide a more sensitive measure of lung function, as shown by its correlation with both restriction and gas transfer issues in our patients.
Our study assessed survivors of severe COVID-19-related ARDS using a progressive exercise test, revealing that nearly half of them suffer from moderate to severe functional disability in the medium term. This distinguishes them from ARDS survivors of other causes who typically experience more severe and enduring declines in function, primarily due to persistent muscle and wasting.36 Notably, the functional limitations observed in our patients seem related to respiratory mechanics impairment, indicated by reduced tidal volume and less pronounced EELV reduction, along with impaired gas exchange shown by elevated carbon dioxide ventilatory equivalent and heightened symptomatic perception during exercise. Additionally, cardiac limitations may contribute, as indicated by differences in peak oxygen pulse and cardiovascular response to exercise.
Furthermore, it is also interesting to delineate the clinical and functional factors associated with decreased exercise tolerance. The presence of a restrictive disorder, characterized by reduced functional residual capacity, impaired gas exchange membrane component, and heightened lung parenchymal density are independently associated with moderate-to-severe disability. The contribution of hypothyroidism may extend beyond its effect on DLCO, encompassing its recognized influence on peripheral muscle function as well.37 Additionally, the association with female sex may be attributed to its established status as a risk factor for post-COVID-19 pulmonary fibrosis.38
In any case, in our opinion, the most significant finding of our study lies in the potential utility of progressive exercise testing and the pulmonary diffusion membrane component in identifying the risk of persistent functional sequelae of COVID-19-ARDS, which characterize the development of pulmonary fibrosis. In survivors of severe COVID-19, it has been described that the mid-term reduction in the most commonly assessed functional parameters (TLC and DLCO) may partially reverse in the long term,29 raises questions about the potential reversibility of post-COVID-19 pulmonary fibrosis mechanisms.39 In our patients, values at 6 months post-ICU discharge of the pulmonary diffusion membrane component, peak oxygen uptake, and two additional exercise response variables (dead space and gas exchange) are independent risk factors for identifying those patients with mid-term TLC or DLCO abnormalities who, at 24 months post-discharge, will exhibit a functional alteration pattern suggestive of pulmonary fibrosis, characterized by a combination of restrictive disorder and gas exchange impairment.
We acknowledge several limitations in our study. Firstly, it is a single-center, observational study. Second, like many other studies, we were unable to assess the premorbid clinical status, making it impossible to rule out the presence of impairments before admission. Third, our patient cohort was collected during two different periods of the pandemic, which could introduce variations in treatments for acute viral infection and differences in the viral variants responsible for lung pneumonia. However, all patients received similar post-COVID-19 management to ensure a consistent evaluation of residual sequelae. Fourth, follow-up assessment was only conducted in patients with functional disturbances 6 months after ICU discharge due to the ethical constraints on repeating procedures in normal subjects. Additionally, CT scans were not repeated during follow-up to avoid excessive radiation. Fifth, there was no control group because it was impossible to simultaneously recruit a similar group of patients with severe ARDS of another origin and given the limitations of using historic control subjects. Sixth, the still small number of studies assessing disability in survivors of ARDS secondary to COVID-19 using cardiopulmonary exercise testing has not allowed us to validate the predictive model described in an external cohort.
In conclusion, this study provides a comprehensive evaluation of the respiratory sequelae affecting survivors of severe ARDS secondary to COVID-19, assessing respiratory symptoms, quality of life, parenchymal and pulmonary circulation alterations, pulmonary function, and exercise tolerance, all in accordance with current standards. Our findings shed light on the extent of medium-term functional disability among survivors, potential underlying mechanisms and determinants, and its utility in identifying persistent functional alterations in the long term.
Funding SourcesThis study was supported by grants from Sociedad Madrileña de Neumología y Cirugía Torácica (NEUMOMADRID), Spain PE20/0001, to F. García-Río, Comunidad de Madrid, Spain SPACE 2-CV-COVID-CM, to F. García-Río and Instituto de Salud Carlos III-Fondos FEDER, Spain COV20/00207, to C. Cubillos-Zapata.
Authors’ ContributionsFG-R had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. JMA and FG-R conceived the study idea. RC, RG, JMA, CC-Z and FG-R contributed to the study conception and design. RC was the project manager. RC, RG, MT-V, SG-T, EA, ED-G, EM-C, MG-G, IT, MN-F, AF-V, MF-V, JMA, CC-Z and FG-R handled recruitment, data collection, deliverance of intervention, and assessment of all participants. FG-R, RC, MF-V and JMA managed the data and statistical analysis. FG-R, RC, RG and JMA were responsible for the study management and coordination. RC and FG-R drafted the first version of the manuscript. All authors contributed to interpretation of the data, revision of the manuscript, and approved the final manuscript and submission of the article.
Conflict of InterestThe authors declare not to have any conflicts of interest that may be considered to influence directly or indirectly in the content of the manuscript.
Data Availability StatementAll the individual patient data collected during the study will be shared. The data will be made available within 12 months of publication. All available data can be obtained by contacting the corresponding author (fgr01m@gmail.com). It will be necessary to provide a detailed protocol for the proposed study, to provide the approval of an ethics committee, to supply information about the funding and resources one has to carry out the study, and to consider inviting the original authors to participate in the re-analysis.
Artificial Intelligence InvolvementNone of the materials have been partially or wholly produced with the aid of any artificial intelligence software or tool.