Bronchiectasis is a complex and heterogeneous disease. Its pathophysiology is poorly understood, but chronic bronchial infection plays an important role in its natural history, and is associated with poor quality of life, more exacerbations and increased mortality. Pseudomonas aeruginosa, Haemophilus influenzae and Staphylococcus aureus are the most common bacteria related to chronic bronchial infection. Non-tuberculous mycobacteria, fungi and respiratory viruses are also present during clinical stability, and may increase the risk of acute exacerbation. Chronic inflammation is present in bronchiectasis, especially neutrophilic inflammation. However, macrophages and eosinophils also play a key role in the disease. Finally, airway epithelium has innate mechanisms such as mucociliary clearance and antibacterial molecules like mucins and antimicrobial peptides that protect the airways from pathogens. This review addresses how the persistence of microorganisms in the airways and the imbalance of the immune system contribute to the development of chronic bronchial infection in bronchiectasis.
Bronchiectasis is a common chronic respiratory disease, clinically characterized by a syndrome of cough, sputum production and respiratory infections, and radiologically by abnormal and permanent dilatation of the bronchi.1 It is a complex and very heterogeneous disorder in its clinical presentation, severity and treatment response.2,3 It is associated with poor clinical outcomes, including worse quality of life and increased mortality. Its incidence and prevalence has increased in the last 10 years, especially in older age groups.4 Despite this, the pathophysiology of bronchiectasis is still poorly understood.
A dysregulated inflammatory response results in lung damage, abnormal and irreversible dilatation of the bronchi and recurrent respiratory infections.5 Bronchial infection plays a key role in the natural history of bronchiectasis. Infective agents such as bacteria, non-tuberculous mycobacteria (NTM), viruses and fungi, have all been proposed to contribute to the pathogenesis by promoting airway damage, increasing bronchial and systemic inflammation6 and evading host immune responses.7 The establishment of airway chronic infection is one of the crucial components of the pathophysiology. Understanding the molecular mechanisms leading to development of airway inflammation and bacterial persistence in bronchiectasis are essential to find new treatments and to improve the management for this disease.8
In this review, we address different pathophysiological mechanisms related to bronchial infection in bronchiectasis. We describe the most common pathogens of bronchial infections and their defence mechanisms. Cellular defence and airway inflammation during bronchial infections is also discussed, especially focused on neutrophils, macrophages, eosinophils and epithelial cells.
Chronic bronchial infectionChronic bronchial infection is a common event in bronchiectasis and it is associated with poor outcomes.9–11 Several infectious agents such as bacteria, mycobacteria and fungi have been isolated from these patients.12,13 The most common ones are summarized in Table 1.
Most common microbiological agents identified in chronic bronchial infection in bronchiectasis.
| Bacteria: |
| Pseudomonas aeruginosa |
| Haemophilus influenzae |
| Staphylococcus aureus |
| Escherichia coli |
| Streptococcus pneumoniae |
| Stenotrophomona maltophila |
| Non-tuberculous mycobacteria: |
| Mycobacterium avium complex |
| Mycobacterium abscesus |
| Mycobacterium xenopi |
| Mycobacterium simiae |
| Mycobacterium gordonae |
| Fungi: |
| Filamentous fungi |
| Aspergillus spp |
| Scediosporum apiospermum |
| Fusarium and Penicillium geni |
| Yeasts |
| Candida spp |
| Saccharomyces cerevisiae |
| Trichosporon beigell |
| Viruses: |
| Coronavirus |
| Influenza A |
| Influenza B |
| Herpes simplex virus |
| Rhinovirus |
Several definitions of chronic bacterial airway infection have been proposed. A recent consensus document by the Spanish Society of Pulmonology, defines chronic bronchial infection in chronic obstructive pulmonary disease (COPD) patients as the growth of the same potentially pathogenic microorganism in three cultures in one year, separated by at least one month.14 Similarly, the Spanish guidelines of bronchiectasis define chronic infection as three or more consecutive positive cultures of the same organism over a period of at least six months, separated by at least one month.15 Current European guidelines define it as two or more isolates of the same organisms at least three months apart in one year.1
It is important to remark that new sequencing technologies such as 16S rRNA has allowed to study microbiome in bronchiectasis, although is heterogeneous and highly complex.16 Several studies have identified dominant organisms during clinical stability which are concordant with those found using culture-based methods, such as Pseudomonas and Haemophilus.17 A reduction in microbiome diversity, particularly the dominance of Pseudomonas, is associated with greater disease severity, higher frequency and severity of exacerbations, and higher risk of mortality.18 Microbiome might therefore identify subgroups of patients at increased risk of poor outcomes who could benefit from precision treatment strategies. Further research is required to identify the mechanisms of reduced microbiome diversity, their relationship with culture-based bronchial infection and to establish whether the microbiome can be therapeutically targeted.
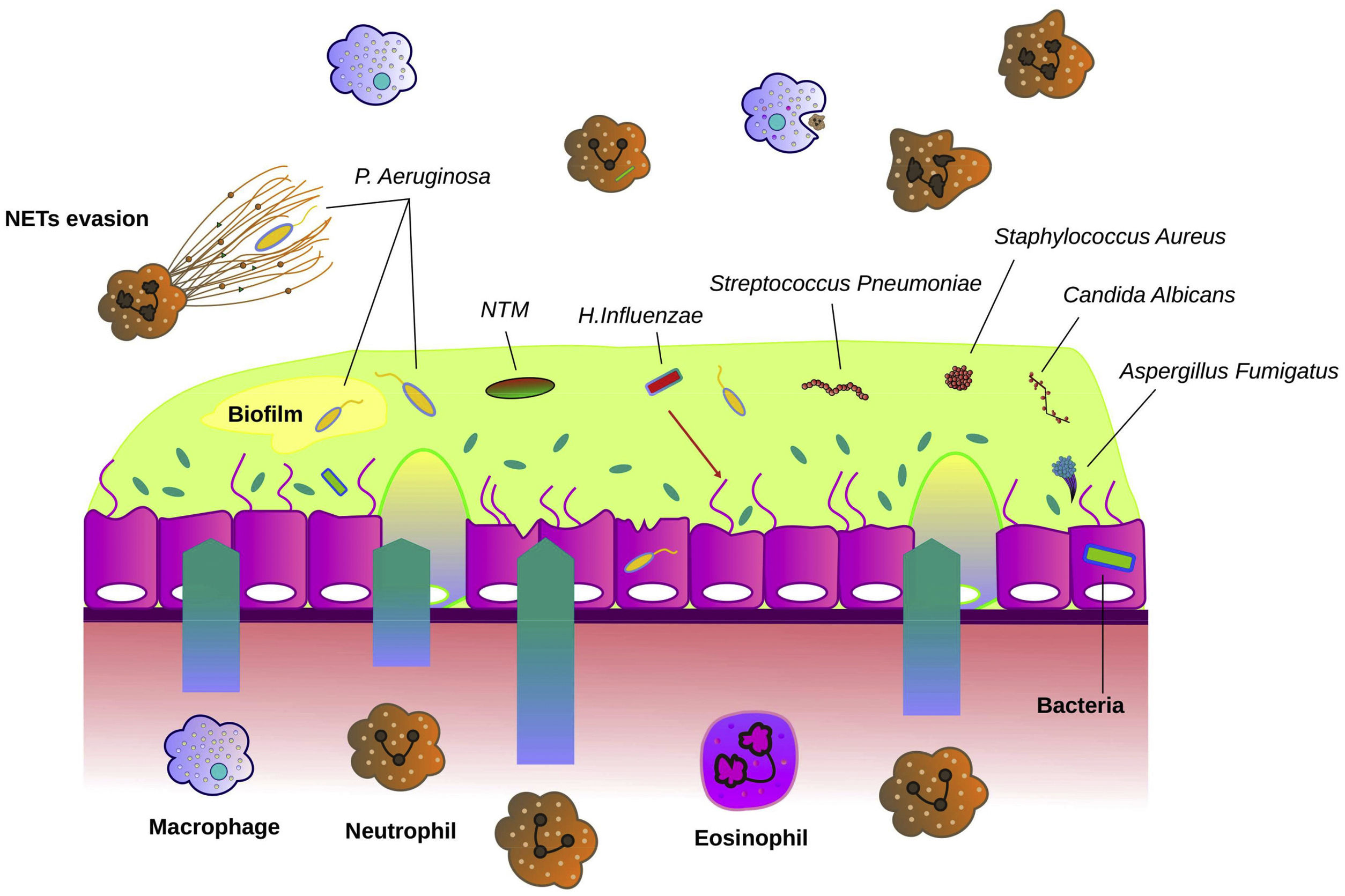
Fig. 1 describes the most common microorganisms related to chronic bronchial infection in bronchiectasis and their interaction with airway immunity.
Interaction between microbiological agents and airway epithelium in bronchiectasis. The bronchial epithelium in bronchiectasis is disrupted and shows increased mucus hypersecretion, especially in the presence of bronchial infection. Bacteria such as Haemophilus influenzae, Streptococcus pneumoniae, Streptococcus aureus and Pseudomonas aeruginosa are often the causative agents of bronchial infections. Fungi such as Candida albicans and Aspergillus fumigatus are other microbes that can cause bronchial infection. Non-tuberculous mycobacteria (NTM) can directly cause bronchiectasis or contribute to worsening previous pathology. The airway epithelium recruits neutrophils, macrophages and eosinophils that migrate into the airways and participate in host defence. P. aeruginosa often forms biofilms to evade the immune system. Another mechanism of immune evasion is bypassing the neutrophil extracellular traps (NETs).
Bacteria are the most common agents isolated from airway secretions of stable and exacerbated patients with bronchiectasis. They include Pseudomonas aeruginosa (PA), Haemophilus influenzae, Moraxella catarrhalis, Streptococcus pneumoniae and Staphylococcus aureus.1 Once the infection is established, it may become chronic with consecutive positive sputum cultures. Chronic infection leads to persistent airway inflammation, thus perpetuating the vicious circle proposed by Cole et al. in 1986.19 However, this model was re-described as a vortex circle since the interactions are much more complex and each pathophysiological step contributes to all the other.20
PA is a pathogenic gram-negative bacterium that causes severe opportunistic infections in immunocompromised individuals and is associated with lung function decline, more exacerbations and increased mortality in bronchiectasis.21,22 Studies examining airway specimens from stable patients have reported PA as one of the most frequently isolated microorganisms (15–50%).23–25
Biofilms are central for PA survival inside the lung. Biofilm protects bacteria from the host immune system as well as increases resistance to antibiotics.26 Neutrophils may inadvertently contribute to stability of biofilms by promoting selection of more resistant strains.27 PA within biofilms becomes non-motile, besides protecting them from phagocytosis. Initiation of biofilm formation is dependent on the process of quorum sensing. As the number of bacteria in the lung increases, concentrations of signalling molecules increase. These diffuse freely between bacteria, allowing organisms to sense local population density. Once a critical mass of bacterial is reached, quorum sensing molecules induce the expression of genes that promote biofilm.28 The hypermutable nature of PA allows it to survive in the lung by expressing or inactivating genes that improve its adaptation to environmental, immune and antibiotic changes.29
Non-typable H. influenzae (NTHi) is a gram-negative organism that can colonize the upper tract of up to 75% normal adults due to its lacks of polysaccharide capsule (distinguishing it from encapsulated forms like type b). It is present in around 5–15% of cultures from stable patients.23,25H. influenzae can infect the airway epithelium and survive intracellularly, resulting in the dysregulation of the host immune response. King et al. hypothesized that recurrent airway NTHi infection may be associated with unclear adaptive immunity. They found that patients with bronchiectasis had a predominant production of Th2 cytokine in response to NTHi infection, a decreased expression of CD40 ligand and a different production of IgG. Therefore, chronic infection with NTHi in bronchiectasis is associated with a change in adaptive immunity that may be important in the pathogenesis of bronchial infection.30
S. aureus is a gram-positive coccus which is occasionally isolated in patients with bronchiectasis (5–15%), but it is more frequently associated with allergic bronchopulmonary aspergillosis and atypical variants of cystic fibrosis (CF).25,31 Similar to PA, it regulates virulence factors during conversion to chronic infection and can form biofilms.32 The ability to form small colony variants is an important feature of S. aureus virulence, but their role in bronchiectasis is unknown. Under anaerobic conditions, which can occur in poorly ventilated areas of the lung, S. aureus forms a polysaccharide intercellular adhesin that protects cells from neutrophil phagocytosis.33
Non-tuberculous mycobacteriaNTM are intracellular pathogens ubiquitously found in the environment and frequently detected in soil and water samples. Different studies have reported a high incidence of these microorganisms, especially in studies conducted in the United States where the prevalence varies from 50 cases of NTM/100,000 people to >200/100,000 people.34 A recent systematic review estimated the overall NTM prevalence in adults without CF bronchiectasis to be 10%. The most prevalent NTM isolated in patients with bronchiectasis are Mycobacterium avium complex followed by Mycobacterium simiae and Mycobacterium gordonae.35–36
NTM can potentially cause bronchiectasis directly or by deteriorating the established pathology.37 This occurs through two main mechanisms. The first one is related to the chronic granulomatous inflammation. This inflammation causes weakening of the airway walls and leads to ulceration and atrophy of the mucosa. The second mechanism involves the formation of chronic inflammatory mucus plugs that cause airway obstruction and dilation. The inability of the lungs to clear NTM triggers an exaggerated inflammatory response through the release of inflammatory cytokines and chemokines by airway cells. Recruited neutrophils release elastase and metalloproteinases that perpetuate airway damage. Elastase prevents opsonisation of mycobacteria and reduces their recognition by neutrophils, leading to reduced clearance of bacteria and promoting NTM survival in the lungs.38
FungiImpaired mucociliary clearance and thick mucus lead to the persistence of fungal spores in bronchiectasis airways, which are constantly exposed to environmental fungi. Aspergillus spp. and Candida spp. are the most frequently isolated fungi in these respiratory secretions.12
Aspergillus fumigatus is recognized as an important airway colonizer and remains the most widely identified fungus associated with bronchiectasis. The epidemiology, pathogenesis, diagnosis, and treatment of Aspergillus-associated disease in the context of bronchiectasis are largely unknown.12 Depending on the underlying host immunity, Aspergillus can cause direct pulmonary damage leading to bronchiectasis or it can alternately trigger a spectrum of syndromes that worse pre-existing bronchiectasis.39
Other species of filamentous fungi found in bronchiectasis include Scedosporium apiospermum and species of the genera Fusarium and Penicillium. Fungi of the family Mucorales, such as Rhizopus spp. and Mucor spp. have also been found. Recently, dematiaceous fungi, such as Alternaria spp. and Bipolaris spp. have been described in bronchiectasis, whose presence is associated with allergic stimuli of the bronchial airways.40,41 Their impact on the natural history of bronchiectasis is not well established, and further studies are needed to better understand their role as physiological or pathological agents in bronchiectasis.
VirusesViruses are recognized as common infectious agents potentially responsible of acute exacerbations in bronchiectasis. However, their role in stable bronchiectasis is not clear. The association between viral infection and bacterial superinfection is well described in other airway diseases such COPD and CF, and could induce important changes in the microbiome.42 In bronchiectasis, recent studies have demonstrated that respiratory viruses are commonly detected during stability. The most prevalent viruses detected were coronavirus, influenza A, influenza B and virus Herpes simplex.43,44 In these studies, their presence was related to increased risk of exacerbations. However, further investigation using prospective studies is needed to elucidate their impact on the disease.
Chronic inflammationChronic inflammation is an essential component of the pathophysiology of bronchiectasis. Patients present extensive airway cell infiltration, especially in severe disease. The inflammatory response involves a complex cytokine network that activates and recruits cells involved in host defence. An imbalance between pro- and anti-inflammatory signals leads to a self-perpetuating inflammatory cycle.45
Inflammatory cellsNeutrophilsNeutrophils are among the first immune cells to be recruited in response to an infection and are considered as key components in the pathophysiology of bronchiectasis.
Neutrophils are recruited to the lung by interleukin-8 (IL-8), IL-1β, IL-17, leukotriene B4 and tumour necrosis factor-α (TNF-α). All these inflammatory mediators are released from the bronchial epithelium to activate neutrophils. Once activated, they initiate the production of reactive oxygen species (ROS) and neutrophil granule products such as myeloperoxidase, neutrophil elastase (NE), heparin binding protein, resistin and matrix metalloproteinases, which are accumulated into the airways, perpetuating the chronic inflammation.46
Neutrophil phagocytosis is dependent on Fcγ and complement receptors. Fcγ receptors recognize phagocytic targets opsonised with IgG, while complement receptor recognizes the complement components (C3b/C4b) deposited on microorganisms and complement receptor 3 recognizes complement component iC3b.7 It is clear that neutrophils from bronchiectasis airway fail to effectively phagocytose and kill microorganisms. However, the mechanisms are poorly understood. Previous studies have not found evidence of reduced phagocytosis by peripheral blood neutrophils in patients with bronchiectasis, suggesting that neutrophils may be normal before they enter into the inflamed airway.47 Interestingly, a recent study demonstrates different phenotypic characteristics according to neutrophil count in patients with stable bronchiectasis. Patients with high number of systemic neutrophils have more severe disease, defined by impaired lung function and greater systemic inflammation.48
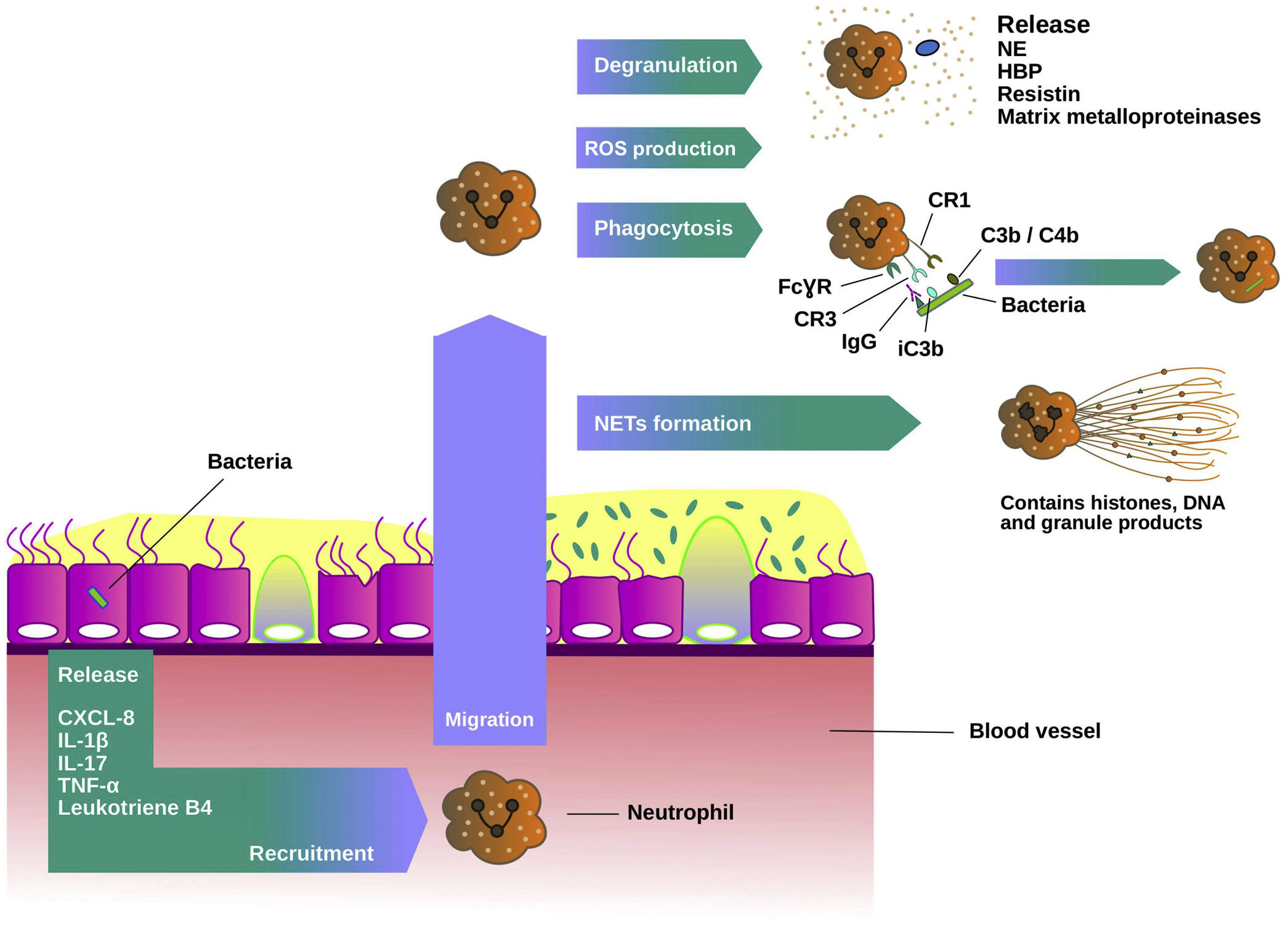
Neutrophil extracellular traps (NETs) are released by neutrophils in response to multiple stimuli including bacterial infection. NETs contain antimicrobial neutrophil granule proteins including NE and histones which are toxic to microbes. A recent study by Keir et al. demonstrated that NET-associated proteins were the most abundant proteins in sputum and they were strongly related to disease severity.49 Neutrophil recruitment process and host defence mechanisms are shown in Fig. 2.
Neutrophils function in chronic bronchial infection. Neutrophils are recruited from the blood to the airways by mediators such as interleukin 8 (IL-8 or CXCL-8), interleukin 1 beta (IL-1β), interleukin 17 (IL-17), tumour necrosis factor alfa (TNFα) and leukotriene B4. In the lungs, they are activated and migrate into the site of the infection. There, neutrophils produce reactive oxygen species (ROS) and release granule products during the degranulation, such as myeloperoxidase, neutrophil elastase (NE), heparin-binding protein, resistin and matrix metalloproteinases, and initiate various host defence mechanisms. Phagocytosis is dependent on Fcγ receptors and complement. Fcγ receptors recognize IgG-opsonised phagocytic targets, while complement receptor 1 recognizes C3b/C4b components deposited on microorganisms, and complement receptor 3 recognizes the complement component iC3b. The formation of neutrophil extracellullar traps (NETs) is another host defence mechanism. NETs contain histones, DNA and granule products to exert their antimicrobial activity.
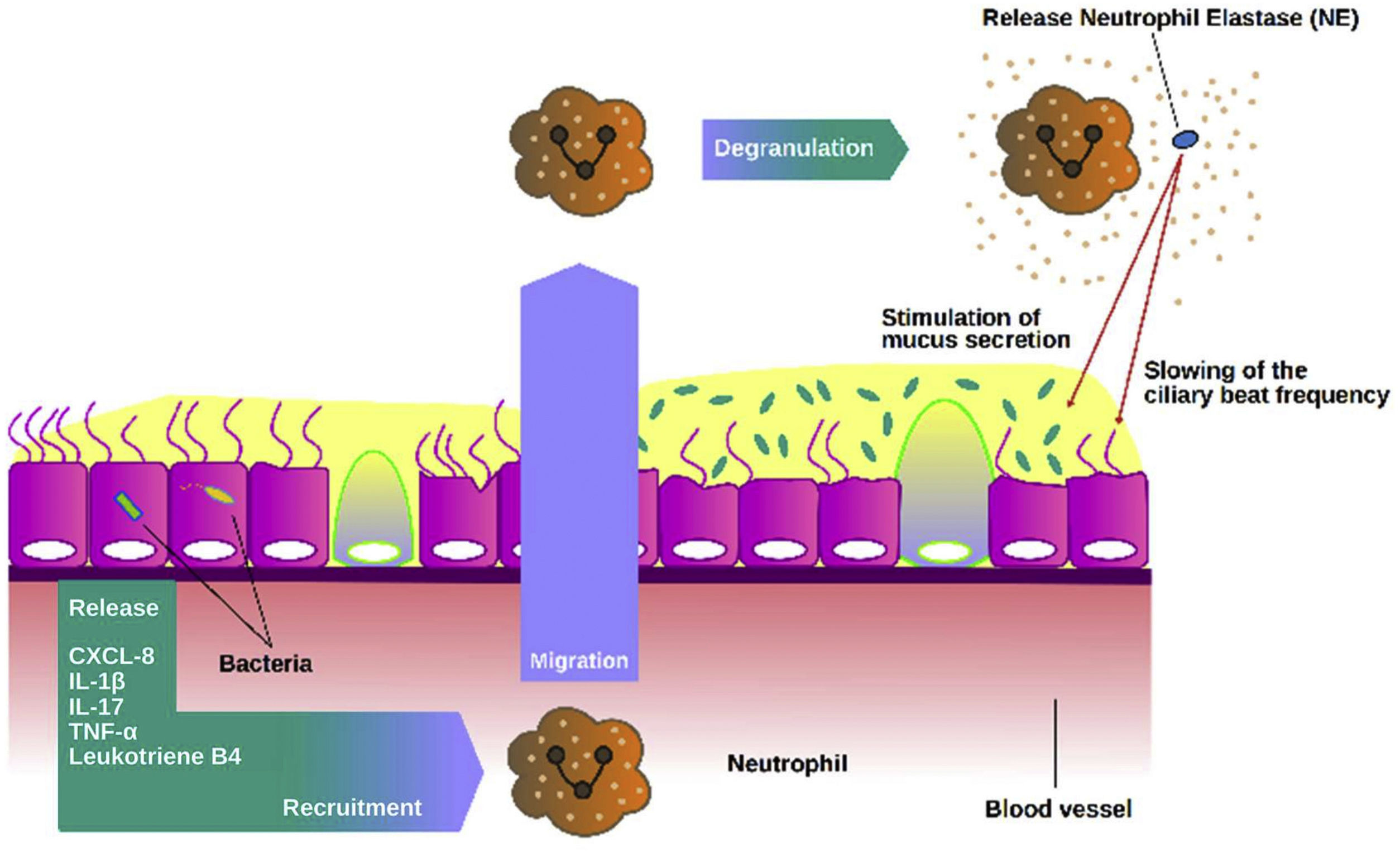
NE is probably the most promising biomarker assessed in sputum in bronchiectasis to date. NE is a 29kD serine protease stored in azurophilic granules that may be released during degranulation, NETs formation or cell death. NE has proinflammatory role, slows cilia beat frequency and stimulates mucus secretion.7,50 Clinical studies have shown a strong association with markers of disease severity, increased bacterial load, number of exacerbations and mortality.51–53 NE functions in bronchiectasis are summarized in Fig. 3.
Role of neutrophil elastase in the airways. Once activated neutrophils are recruited into the airways, they release high amounts of neutrophil elastase (NE). NE can stimulate mucus secretion as well as slow the frequency of ciliary beating, impairing the mucociliary epithelium and making it more susceptible to chronic infection.
Macrophages are critical in immune response for the detection, phagocytosis, and eradication of pathogens as well as the initiation of the inflammatory response through cytokine release.54,55
The clearance of apoptotic cells by macrophages, called efferocytosis, is also a key mechanism for the resolution of inflammation. Impaired efferocytosis is associated with increased inflammation and airway damage with secondary necrosis and release of granulation products. In bronchiectasis, the role of macrophages has been less studied, but it is known that the number of macrophages in biopsies from patients with bronchiectasis are increased, although their function is altered.54
EosinophilsBronchiectasis is classically defined by neutrophilic inflammation, but bronchial biopsies have shown increased eosinophil infiltration in a subset of patients.56 A recent study from Shoemark et al. has described a relationship between sputum and blood eosinophil counts in bronchiectasis. This study also showed that blood eosinophilia (>300cells/μL) was present in 20% of bronchiectasis cases and that it was strongly associated with Streptococcus- and Pseudomonas-dominated microbiome profile and with shorter time to next exacerbation.57 Another study using the Spanish Online Bronchiectasis Registry (RIBRON) showed that those patients with eosinophils higher than 100cells/μL had significantly better clinical outcomes, lung function, and nutritional status, while showing lower systemic inflammation levels.58 Some authors have observed that inhaled corticosteroids could be beneficial in these patients with eosinophilic bronchiectasis.59 Further studies are needed to clarify the role of eosinophils in bronchiectasis, which may be a potential biomarker that can help to identify individuals who require different management strategies.
Airway epithelial inflammationRespiratory epithelium has several innate mechanisms such as mucociliary clearance, secretion of antibacterial molecules or mucus content that constantly defend the airway against pathogens.
Epithelial cellsMucociliary clearance is the main function of bronchial epithelial cells and protects airways from bacterial infections. Cilia move in a coordinated fashion to clear mucus accumulation, avoiding the establishment of bacterial infections. Epithelial cells also release proinflammatory mediators which trigger neutrophil migration to the site of infection.60,61
One of them is the potent vasoconstrictor endothelin-1 (ET-1). It has a wide range of biological activities in the respiratory tract,62,63 such as promoting neutrophil adhesion to endothelial cells and migration to areas of inflammation. Several studies even suggest a significant pathogenic role for ET-1 among PA infected patients with bronchiectasis.64 Increased cell surface expression of the adhesive glycoprotein for leukocytes intercellular adhesion molecule-1 (ICAM-1) is another mechanism for epithelial cell regulation of the airway response to bacterial pathogens.65 ICAM-1 leads to increased adherence of neutrophils in airway epithelial cells through neutrophil surface receptors CD11/CD18. This improves phagocytic functions and promotes increased inflammation.66
Airway mucosal immunity also involves the presence of immunoglobulins to prevent the adherence of bacteria to the epithelium. Epithelial cells may express immunoglobulin receptors to allow the release of the dimeric form of secretory IgA, the main immunoglobulin found in the airways.67 Primary antibody deficiency and acquired immunoglobulin deficiencies associated with haematological malignancy can cause bronchiectasis. Failure to produce specific antibody responses against specific pathogens may be even described in patients with normal total IgG levels.68
Antimicrobial peptidesAntimicrobial peptides (AMPs) are important in pulmonary host defence against pathogenic microbes.69 The most abundant airway AMPs are lysozyme, lactoferrin and cathelicidin LL-37 (which are proinflammatory mediators released from neutrophils, macrophages and airway epithelium) and secretory leucocyte protease inhibitor (SLPI) produced by cells from bronchial epithelium and with anti-inflammatory functions.70 A prospective study suggested that patients with more severe disease at baseline had a dysregulation of airway AMPs. Especially higher LL-37 and lower SLPI levels were associated with Bronchiectasis Severity Index, lower FEV1 and PA infection. Low SLPI levels were also associated with exacerbation frequency at baseline. During follow-up, higher LL-37 and lower SLPI were associated with a shorter time to exacerbation, whereas LL-37 alone predicted exacerbation frequency over the next 1-year.71 Cluster analysis allow the identification of clusters (endotypes) based on different sputum AMPs levels.72
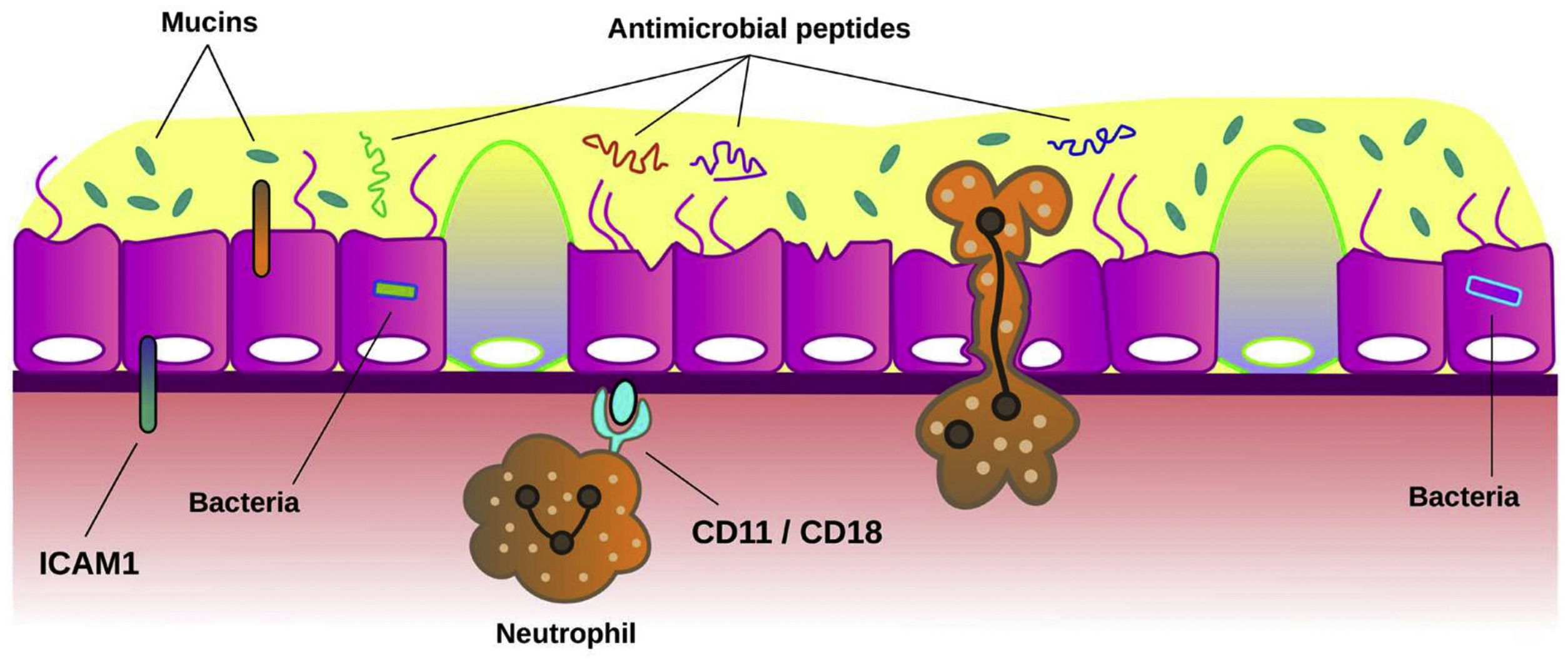
MucinsMucins are the major macromolecular component of the mucus gel in healthy conditions.73 They are glycoproteins responsible for the mucus protection and clearance. MUC5AC and MUC5B are the major secreted mucins detected in sputum. Experimental studies confirmed the crucial role of secreted mucins for airway defence.74 Several studies have shown that mucin concentration is significantly higher in patients with bronchiectasis than in healthy subjects and it has been related to airway inflammation and bacterial load.75 Recently, it has been demonstrated that bronchiectasis sputum exhibited increased percent solids, total and individual mucin concentrations, osmotic pressure, and elastic and viscous moduli, suggesting that hyperconcentrated airway mucus is characteristic in bronchiectasis and that may be a target for pharmacotherapy.76Fig. 4 summarizes the role of the airway epithelium against airway infection.
Mechanisms of airway epithelium against bronchial infection. Mucociliary clearance is the main function of airway epithelium against bacterial infection. When the function of cilia from ciliated cells is impaired or the concentration of mucins is altered in mucus, bronchial infection can be easily established across the epithelium. Also, bacteria induce the expression of intercellular adhesion molecule 1 (ICAM-1) on epithelial cells. This molecule plays multiple roles in modulation of inflammation such as inducing neutrophil adhesion to airway epithelial cells through neutrophil surface receptors CD11/CD18. The release of antimicrobial peptides from epithelial cells and neutrophils is also a key host defence mechanism to limit infection.
Chronic bronchial infection by bacteria, mycobacteria, fungi and/or viruses is common in bronchiectasis and has been related to worse clinical outcomes. The persistence of an infectious agent in the airways of patients with bronchiectasis is the result of multiple defects in the immune response and different microorganisms defence mechanisms, which leads to the development of chronic bronchial infection. Although significant progress has already been made, further studies focusing on epidemiology, risk factors, microbiological agents, and clinical relevance in relation to inflammatory endophenotypes are needed. Understanding the pathophysiology of chronic bronchial infection is essential to personalize future treatments and to improve the management of patients with bronchiectasis.77
FundingThis study has been funded by Instituto de Salud Carlos III (ISCIII) through the project “PI21/00419” and co-funded by the European Union. This work has also been funded by Contractes Clínic de Recerca “Emili Letang – Josep Font” 2021 (Belen Solarat).
Conflict of interestThe authors declare to have no conflict of interest directly or indirectly related to the manuscript contents.
The authors would like to thank Xose Quiroga for his collaboration in the creation of the figures.