Introduction
Emphysema is characterized by the destruction of alveolar walls and an abnormal, irreversible increase in the size of alveolar spaces that lead to hyperinflation, loss of lung elasticity, and reduced expiratory flow. As a consequence of such morphological changes in the lung, structural changes also occur in the chest wall, particularly in respiratory muscles.
The diaphragm, the main respiratory muscle, is flattened, its dome descending so that fibers become shortened. The diaphragm is then less able to generate tension, such that the metabolic requirements are greater for a given workload.
Alterations are found not only in respiratory muscles of patients with emphysema: peripheral muscles also undergo functional changes. This has been defined as the systemic myopathy of chronic obstructive pulmonary disease.1 These muscle alterations are attributed to multifactorial causes (mechanical overload, nutritional changes, hypoxemia, pharmacological effects, etc).2
In patients with emphysema, respiratory and peripheral muscle changes play a large role in most symptoms, such as weakness, pain, and fatigue.3-5 This is partly shown by the low correlation between lung function and exercise capacity,6 quality of life,7 prediction of severe exacerbations,8 or patient survival.9,10 In addition, the functional improvement attained with medication is not accompanied by significant improvement in exercise capacity,11 and improved walking test performance observed after lung transplantation is very similar for single- or double-lung transplants, in spite of the superior function afforded by a double-lung transplant.1,12
In the presence of severe lung distension, thoracic cage deformation and morphological changes of the diaphragm occurring in very advanced stages of emphysema, lung volume reduction surgery (LVRS) can be considered with the object of reducing lung distensibility, improving elastic lung forces,13 recovering the optimal position of the diaphragm, and improving the position of intercostal muscles to increase inspiratory pressures.14 The contribution of abdominal muscles is also increased.15
Pathophysiological Changes After LVRS
LVRS removes clearly destroyed areas of the pulmonary parenchyma, so that residual volume (RV) and total lung capacity (TLC) decrease while vital capacity and forced expiratory volume in 1 second (FEV1) increase.
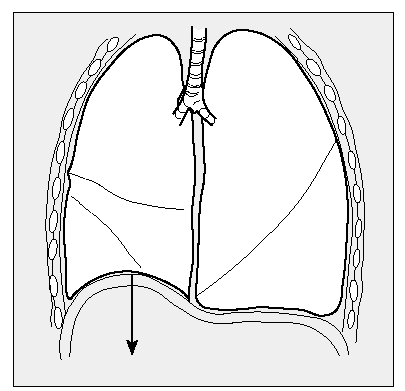
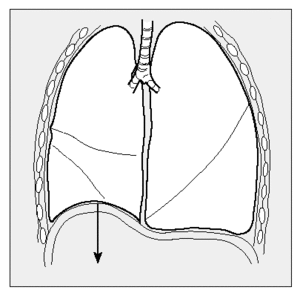
These changes in lung mechanics are caused by the increase in elastic lung forces13,17,18 that oppose the expansive force of the chest wall, so that the tendency to overdistension of the chest is reduced and the dome of the diaphragm is raised (Figure 1), the zone of apposition to the rib cage increases,19 as do expiratory flow and inspiratory conductance of the airway; traction force around the airway also improves.13,20-22
Figure 1. LVRS reestablishes the position of the diaphragmatic dome, which becomes lower in patients with emphysema.
These pathophysiological changes in the respiratory system lead to an improvement in dyspnea and exercise capacity.23 Respiratory mechanics improve when the diaphragmatic function is enhanced and every inspiratory muscle is recruited, both when the subject is at rest and exercising. This improvement in exercise capacity brings about the reduction in respiratory work and the increase in maximum voluntary ventilation observed after LVRS.14
LVRS improves the overall strength of inspiratory muscles, increasing inspiratory muscle and trans diaphragmatic pressures.13,24-27 The improvement in diaphragmatic function can be partly explained because the remodeling of the thoracic cage--which causes a reduction in its anteroposterior and transversal diameters--increases the length of the diaphragm, its vertical zone, and the zone of apposition to the rib cage. Remodeling also raises the dome.
Moreover, LVRS not only acts by modifying respiratory muscles, but may also cause certain changes in peripheral muscles, which are also affected in patients with emphysema. Peripheral muscle function improves when the blood supply needed by respiratory muscles decreases and oxygen transport is enhanced.28 This may partly explain the improvement observed in the walking test performance of patients who have undergone LVRS, even though FEV1 improves only slightly.29
Outcome of Surgery
After more than 8 years of experience with LVRS, questions about its usefulness remain and the variables that best demonstrate the procedure's success (FEV1, dyspnea, walking test, etc) have not been agreed upon. Nor is there unanimity on the morphological or pathophysiological variables that should be used to select the most appropriate candidates, or the degree of improvement that should be expected for each analyzed variable in order to define success or failure of the treatment.
The first reports of studies with short-term follow up observed an increase in FEV1 from 20% to 70%, depending on surgical technique and patient characteristics.30-34 The improvement in FEV1 was accompanied by an increase in exercise tolerance and by reduced TLC and RV (Table).
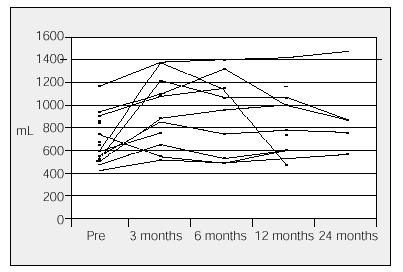
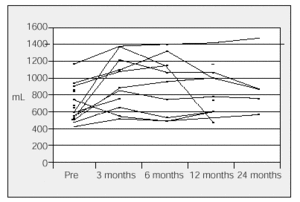
In our hospital, 20 patients who had surgery between 1996 and 2000 showed a significant increase in FEV1 (from 86 to 871 mL) (Figure 2). The increase of more than 200 mL was reached in 56% of patients who had surgery and was maintained for two years in 30% of the cases.35 There was a significant improvement in dyspnea (7±1 vs 3.2±1; P<.005) and the walking test (238±70 vs 346±62 m; P<.01) in the year of surgery. Generally, lung function reaches a maximum value between 3 and 6 months after surgery but then decreases over time.
Figure 2. Results of LVRS in Clínica Puerta de Hierro in Madrid. Evolution of forced expiratory volume in 1 second (FEV1) in each patient who had surgery. Mean increase in FEV1: 41%. Increases between 86 and 871 mL.
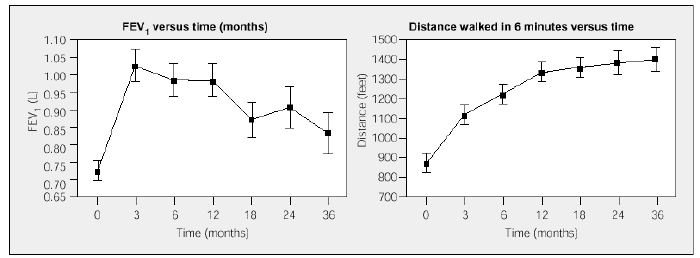
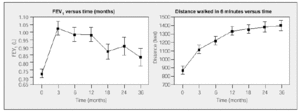
Long-term follow-up studies have confirmed the clinical and functional benefits of LVRS observed during the first 6 months, in spite of later deterioration of lung function.29,36-38 In one third of the cases, functional improvement is maintained beyond 4 years after surgery; in patients with functional decline, the improvement attained in some series with the walking test is maintained and even increases over time29 (Figure 3).This data supports the hypothesis that LVRS has positive effects on overall muscle function of emphysematous patients and that these effects last more than those on the lung itself.
Figure 3. Forced expiratory volume in 1 second (FEV1) and walking test results after LVRS. Postoperative walking test performance improves over the long term in spite of the detected deterioration in lung function (from Flaherty et al).29
Although no properly designed randomized trials have been published comparing LVRS with medical treatment and respiratory muscle training--so we cannot say that there is complete evidence in support of LVRS--trials comparing patients with emphysema under medical treatment and patients with emphysema undergoing surgery have been published. In patients who had surgery, significant improvements in FEV1 (between 23% and 53%), exercise tolerance, intensity of dyspnea and quality of life were observed, though it should be taken into account that these studies enrolled small numbers of patients and that follow up was short-term (less than 12 months).40-42
The detailed analysis on the functioning of respiratory muscles in patients with emphysema undergoing LVRS shows that esophageal and gastric pressures have been reduced, both at rest and exercising, three months after surgery.14 Inspiratory muscle pressure and trans diaphragmatic pressure increase after LVRS, if compared to the values measured at the end of the rehabilitation program.18,26 The observed improvement in respiratory muscle function is greater when exercising than at rest.18 Both the length of the diaphragm and the capacity to generate transdiaphragmatic pressure are reduced in patients with emphysema, in comparison with normal subjects. After LVRS, normal values are recovered when functional residual capacity is determined, though the TLC remains low according to Bellemare et al.43 Those same authors noted that the structural changes that LVRS produces in the chest cavity only affect the length of the diaphragm, not the position of the rib cage or the dimensions of the thorax. This indicates that surgical treatment brings about an adaptation of the diaphragm which improves its ability to contract, possibly because of changes in sarcomeres, as demonstrated in the first experimental studies on animals, in which LVRS increased the number of sarcomeres in the diaphragm.44
Measured changes in muscle function are related to clinical changes observed after LVRS. The improvement in dyspnea correlates with the decrease in the esophageal pressure.18 In a multiple regression analysis, increased inspiratory muscle pressure is the parameter that correlates best with reduced RV. A correlation between the reduction in VR and TLC and the increase in diaphragm length has also been observed, mainly in the zone of apposition to the rib cage.45
Together, these observations indicate that LVRS leads to better recruitment of inspiratory muscles, especially of the diaphragm. More optimal respiratory muscle functioning could explain the improvement in dyspnea and exercise capacity of patients who have had surgery, in spite of the small change observed in expiratory flow. However, all studies on muscle function enroll small patient groups and have only short-term follow up, so it is not yet known whether or not these changes are maintained over time.
Studies on quality of life show an improvement of the various aspects analyzed in the questionnaires used.46,47 Although respiratory muscle training carried out before surgery produces physical improvements, surgery itself brings about improvements in psychological aspects and vitality as well.48 Leyerson et al49 observed that the improvement in quality of life correlated with a reduction in the RV/TLC ratio, increased oxygen uptake, and reduced use of corticosteroids.
Surgical teams with extensive experience of LVRS, having carried out more than 100 procedures, present an operative mortality between 0% and 8%, mainly due to respiratory failure, surgical bleeding, and persistent air leaks. The largest series that has been published reported a 1-year survival of 96%, a 2-year survival of 81%, a 3-year survival of 69%, a 4-year survival of 54%, and a 5-year survival of 42%.38 Most of these long-term deaths are caused by respiratory failure due to the progression of the emphysema.
Predicting Response to Treatment
Functional improvement attained with LVRS varies highly from patient to patient, so researchers have looked at various selection criteria that might predict a candidate's response to surgery. When analyzing morphological criteria, a positive correlation has been observed between the heterogenicity of the emphysema and its predominance in the superior lobes on the one hand and functional improvement and improved walking test performance on the other.50,52 Nevertheless, morphological variables have scarce negative predictive value (63%) for functional improvement, and following those findings surgery in all patients with homogeneous emphysema would be ruled out.29 Another problem for selecting candidates is the methodology used to classify and quantify the heterogenicity of the emphysema. While some groups use pulmonary scintigraphy for visual inspection or apply ratios between apical and basal perfusion scans, other groups measure heterogenicity by high resolution computed axial tomography.52,53
Certain functional criteria, such as inspiratory resistance and inspiratory conductivity, have been related to greater postoperative increase in FEV1. However, it seems that the combination of morphological and functional criteria would better predict response to treatment. Thus, Ingenito et al54 observed that by combining a heterogenicity score measured by perfusion scanning and a score for airway conductivity, they were able to select a subgroup of patients with homogeneous emphysema and little intrinsic airway involvement (measured by inspiratory resistance <10 cm H2O/L/s) who would experience benefit from surgery similar to that observed in patients with heterogenous emphysema.
The initial analysis of the National Emphysema Treatment Trial (NETT) comparing medical treatment and respiratory muscle training in patients with emphysema who had undergone LVRS has identified a group of patients whose improvement is negligible and whose operative mortality is high (16%). Criteria that correlate with high surgical risk are a FEV1 less than 20% associated with carbon monoxide diffusion less than 20% and/or homogeneous emphysema.56 Poor carbon monoxide diffusion was already identified as a risk factor based on previous publications,57,58 but its role could not be confirmed in all cases.58-60 What is clear from these publications is that not all patients with emphysema have the same response to LVRS, as reflected in differences in operative mortality and clinical and functional improvement.
Conclusion
To conclude this analysis on LVRS in patients with emphysema, we can affirm that this surgical technique should be considered a therapeutic option for some patients with severe emphysema and a markedly diminished quality of life and exercise tolerance.
One of the main aspects to bear in mind is the degree of lung inflation that impairs the contraction of the diaphragm. After surgery, the patient's lung function and general muscle function improve as inspiratory muscle recruitment increases. Measured changes of muscle function correlate with observed changes in ventilatory mechanics and exercise capacity.
LVRS benefits some patients with severe emphysema not only by reducing the most damaged parts of the lung but also by globally improving respiratory system physiology, ventilatory mechanics, and muscle function.
It remains to be specified how patients should be selected to guarantee the optimal, lasting physiological changes that mean longer-lasting clinical improvement.
Correspondence: Dra. Alicia de Pablo.
San Martín de Porres, 4. 28035 Madrid. España.
Manuscript received March 24, 2003.
Accepted for publication April 1, 2003.