Introduction
Cystic fibrosis (CF) is the most common terminal genetic illness amongst Caucasians. The median survival age has increased more dramatically over the last 25 years than for any other chronic illness,1 reaching 33.4 years by 2001, according to the American Cystic Fibrosis Foundation.2 The improvement in survival of CF patients has meant that the number of CF patients over the age of 18 years has increased from 8% in 1970 to 39.5% in 2001, in the USA.2
Exacerbations and hospitalization increase after the first ten years of CF patients' lives, so patients and their families are familiar with many treatment effects and there is no reason why treatment should not be carried out in the patient's home.
According to data from the American Cystic Fibrosis Foundation for 2001, cultures of respiratory secretions were positive for Pseudomonas aeruginosa in 58.8% of cases, Staphylococcus aureus in 48%, Burkholderia cepacia in 3.1%, and Stenotrophomonas maltophilia in 8.4%. The only data available for Spain were collected in 1997 by the Federación Española contra la Fibrosis Quística. Only 15 CF units out of the whole country participated in this registry, which included a total of 823 patients with a median age of 15.1 years. Microbiological findings for these patients revealed that 37.06% were colonized with P aeruginosa, 35.60% with S aureus, 12.88% with Haemophilus influenzae, 2.79% with S malthophilia, 1.94% with B. cepacia, and 15.9% were not colonized by any germ.
Intravenous (IV) antibiotic treatment is normally given to patients with moderate to severe respiratory exacerbations, or in cycles for patients chronically colonized by P aeruginosa, mucoid phenotype. In the latter case, adverse effects such as allergic reactions to antibiotics or increased bacterial resistance to antibiotics are more common.
Nearly 2.5 times more adults than children currently elect home intravenous antibiotic treatment (HIVAT). According to data from the American Cystic Fibrosis Foundation, HIVAT use increased from 12% in 1990 to 20.3% in 2001.2 In order to discover the current situation of HIVAT for CF patients in Spain, the Sira Carrasco Foundation in aid of CF organized a consensus conference for specialists involved in CF, in Madrid on April 26, 2002. Two months prior to the conference, a letter was sent to all the CF units in Spain, saying that on the web page of the Sira Carrasco Foundation (www.fundacionfibrosisquistica.org) there was a questionnaire on HIVAT to be filled in and sent by Internet or post. The data from the questionnaires was statistically processed and the results discussed and consensus reached at the conference. Twenty CF units participated; among them they control 1230 patients, 740 younger than 18 years of age and 490 older.
This document summarizes the consensus reached on different questions related to HIVAT and the resulting recommendations.
Cycles of IV Antibiotics Carried Out at Spanish CF Units
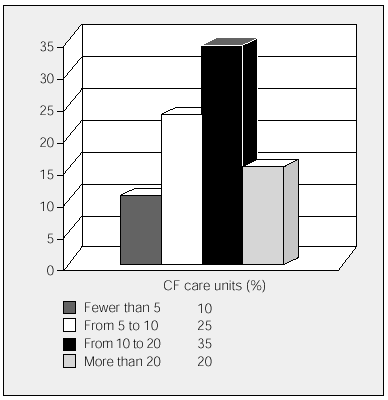
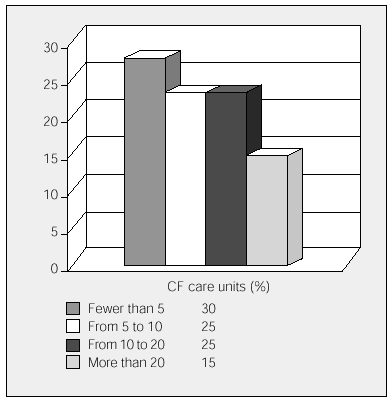
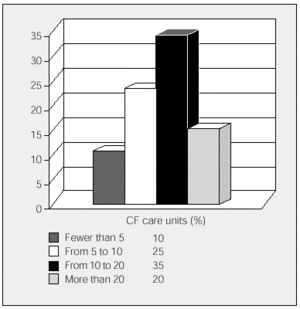
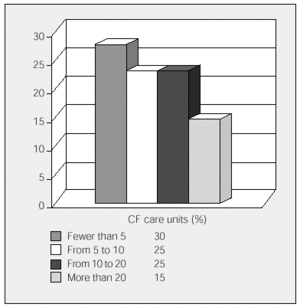
All the participating units have HIVAT programs. The units were asked to state the approximate number of IV antibiotic cycles that were administered annually in the hospital and the number administered in patients' homes (fewer than 5, 5 to 9, 10 to 20, and more than 20). Overall, more cycles were carried out at home than in hospital, although 6 participating units with mainly pediatric populations administered more cycles in the hospital. The data is shown in Figures 1 and 2.
Figure 1. Annual cycles of inpatient intravenous antibiotic treatments carried out by cystic fibrosis units. CF indicates cystic fibrosis.
Figure 2. Annual cycles of home intravenous antibiotic treatments carried out by cystic fibrosis units. CF indicates cystic fibrosis.
Efficacy of HIVAT
In a systematic review of the literature comparing HIVAT with IV treatment in hospital for patients with CF,3 only 1 trial met the selection criteria to demonstrate the efficacy of the therapeutic interventions. That randomized controlled trial,4 enrolling very few patients, found that after antibiotic therapy the lung function parameters (forced expiratory volume in one second, and forced vital capacity) were slightly inferior for HIVAT patients but the improvement in most of the parameters that measured quality of life was greater for the home treatment group. There were also marked economic advantages for the home treatment group and side effects were infrequent in both groups. Well-designed studies need to be carried out, however, to confirm these results.
Medical experience in the use of HIVAT is more extensive for other illnesses and conditions: osteomyelitis, soft tissue infections, endocarditis, neonatal infections, cancer, and pain among others. Well-established programs designed for HIVAT have existed in some countries for several years.5-8 More information on this kind of therapy is available at the OHPAT web page at www.ohpat.org.uk.
Even though the units that answered the questionnaire have carried out no controlled trials, 84% believed that HIVAT was as efficacious as antibiotic treatment administered in hospital.
Therefore, although there is currently no evidence in the literature, HIVAT may be as efficacious as inpatient treatment. Appropriate infrastructure, careful selection of patients, provision of good information, and training and adequate monitoring of the patient are very important for patients that are going to receive this kind of therapy.
Advantages and Disadvantages of HIVAT
The main advantages and disadvantages can be summarized as follows:
Advantages
--Improvement in quality of life, clearly documented in the references.9,10 Patients continue normal life as regards work and studies and have all the benefits of home life, along with more independence and well-being.
--Reduction of periods of hospitalization with the subsequent reduction of costs and risk of infection.11
--Reduced cost with the same effectiveness.12-14 An estimated US $1 000 000 would be saved if only half the CF patients treated annually at a particular hospital received home therapy.10 Many studies have evaluated the cost-effectiveness of HIVAT to its advantage.15
Disadvantages
--Treatment is not as intensive or as standardized as it is in a hospital environment where nutritional assistance, respiratory physiotherapy, and exercise programs are better.
--Adequate monitoring is more difficult.
--Increased burden and psychological stress on the patient and the family.
--Legal-medical problems.
The panel of experts agreed that the advantages outweighed the disadvantages especially considering the improved quality of life for the patient, the reduction in the risk of cross infections from hospitalization, and the reduction in cost involved.
Composition of a HIVAT Program Work Group
The structure of the work group of the HIVAT program must be carefully considered.
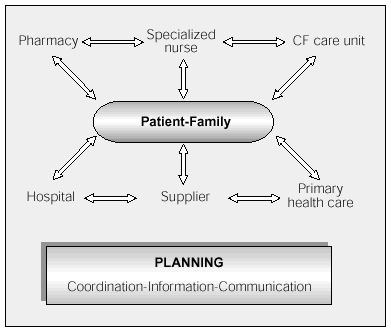
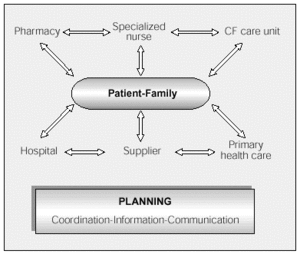
Published studies5,7-9 coincide in that the basic home health care team should consist of a specialized nurse,9 a doctor, and a pharmacist, working together with social workers and primary health care staff (Figure 3).
Figure 3. Structure and organization of the home health care team. CF indicates cystic fibrosis.
The panel of experts recommended that the home health care team should consist of:
--A specialized nurse (from the CF unit, the hospital, the day hospital, or the home support team, or even the primary care center, depending on the circumstances of the CF unit), who should be the coordinator of the program.
--A doctor in charge, normally a specialist in respiratory medicine from the CF unit.
--A back-up group made up of the rest of the components of the unit, a contact with primary health care if necessary, and a pharmacist. Good communication between the members of the group is fundamental.
In the case of illnesses other than CF, the doctor in charge is usually a specialist of infectious diseases, internal medicine or is interested in home care.
Patient Selection
Careful selection of patients is essential to increase the effectiveness of the different procedures of a home health care program.16
The various parameters and situations that should be taken into account when choosing the patients for HIVAT, such as age, distance from hospital, adherence to treatment, and psychosocial problems were considered. The panel of experts found that psychosocial problems (60%) constituted the main obstacle to this therapy. Serious respiratory exacerbations, sepsis, respiratory insufficiency, and moderate to severe hemoptysis together formed an impediment to HIVAT in 45% of cases, while poor adherence to the general CF treatment was reported in 40% of cases. Only 5% of the participating groups considered distance from the hospital to be an impediment to HIVAT, and none considered age to be a disqualifying motive.
The following inclusion and exclusion criteria were defined:
Inclusion Criteria
--Any CF patient with moderate respiratory exacerbation that needed IV antibiotics and who had no criteria for exclusion.
--Programmed, three-monthly, IV therapy (therapy regimen used by Danish and some Spanish units).
Exclusion Criteria
--Serious respiratory exacerbation, at least during the first days until stabilized.
--Respiratory insufficiency.
--Systemic infection.
--Moderate to severe hemoptysis.
--Patients who do not adhere to normal therapy.
--Serious associated psychosocial problems.
--Drug addiction.
HIVAT must always be voluntary, freely chosen by the patient. Age should not be a contraindication. Care must be taken with adolescents as they are at a stage in life when they can show a certain rebelliousness that predisposes them to neglect the treatment. Distance from the hospital should not be more than 2 hours; if longer, control should be left in the hands of the primary care center or local hospital.
Psychosocial characteristics of the patient and family should always be evaluated (characteristics of the home: telephone, running water, refrigeration and adequate hygiene being necessary). Patient and family should be clearly motivated to use the HIVAT program.
Training in the Administration of IV Treatment
The training necessary for a patient to be able to have IV treatment at home, helped or not by the family, will be discussed in the following sections, as it was at the conference.
Staff in Charge of Training
Training in the method of administering antibiotics at the Spanish units is done by the CF unit nurse in 40% of cases, by the day hospital nurses in 25%, by hospital ward nurses in 20%, by the doctor in 10%, and finally by the nurse in charge of the home health care team in 5%.
Thus, training of patient and family will be given by the nurse from the CF unit or nurses from the hospital ward/day hospital or the nurse in charge of home health care according to the characteristics of each unit. Participation of the nurse from the primary care center will be necessary when patients live far from the hospital.
When and Where to Carry Out Training
The patient was hospitalized for the initiation of the antibiotic cycle and then treatment was continued at home in 60% of the participating groups; the first dose only was administered in hospital in 40%.
Patients must be admitted to hospital the first time they are given IV antibiotics. Training is given at this moment and the patient is allowed to go home when the nurse considers patient and family are capable of carrying out the treatment. In subsequent cycles only the first dose need be given in hospital, to rule out possible allergic reactions if different antibiotics are being used. Records must be kept and added to. Patients might be admitted to hospital for 24-36 hours to insure the absence of side effects, measure serum antibiotic levels (peaks and troughs), and evaluate the capacity of patient and family to be trained in the technique. Hospitalization can only be avoided in the first IV antibiotic cycle in some special circumstances, for patients with exceptional learning capacity.
Training Period
The training period should be sufficiently long for the patient and family to feel safe and totally familiarized with the technique. Training time varies from one patient to another and so must be individualized.
Information Supplied
The best way of providing information, and the availability or otherwise of written guidelines on HIVAT was evaluated. Most groups only gave patients oral information and only 40% of the participating hospitals had information leaflets they gave patients on hospital discharge.
The results of the consensus were that the information should be a) clear and concise (oral and/or written); b) via information leaflets; and c) through audiovisual methods that improved communication with patient and family. These methods of information and education should contain the basic concepts of HIVAT, care of catheters and material, preparation of formulae and medication, and cleaning procedures, with descriptions of possible complications or side effects of the drugs or other procedures and ways of resolving them.
Venous Access and Infusion Devices
Venous Access
Most groups use peripheral veins (85%) for antibiotic infusion, with good results; only in special cases is a central venous catheter used (25%) or a central venous catheter with peripheral access (20%). Some recommend the use of central silicon catheters with peripheral access as being well-tolerated and long-lasting, more than 18 months in some cases. This coincided with the opinion of other groups.17
The optimal venous access depends on the characteristics of the patient, accessibility of the veins, and other factors.
According to the literature reviewed,4,5,16,17 a peripherally accessed central vein is recommended, as they can be kept open longer and need less manipulation. Although a peripheral vein is also frequently catheterized, a central vein, with a subcutaneous port, is used when venous access is difficult or frequent cycles of IV therapy are prescribed.
A peripheral vein, then, might be used as the option of choice. If venous access is difficult, or patients need frequent antibiotic cycles, a peripherally accessed central vein can be used. A conventional central vein will only be catheterized in exceptional cases where there is no adequate peripheral access.
Infusion Devices
The device most commonly used by the participating groups was the Intermate (Baxter, Deerfield, Illinois, USA) in 55% of cases, followed by gravity-fed drips in 45%. Maxx 100 (Baxter, Deerfield, Illinois, USA) was used in 15% of cases and direct drug infusion in 10%.
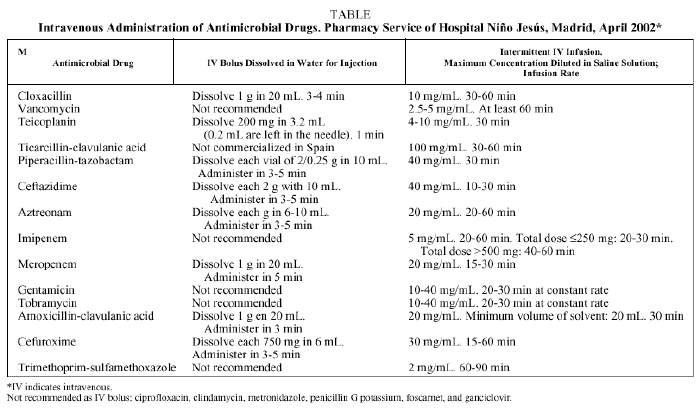
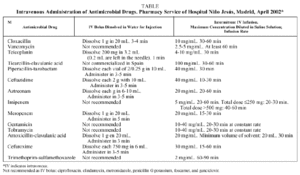
Conventional devices (gravity-based drips) are easy to use. Special devices18 (Intermate, Maxx 100) allow the patient more independence and mobility during antibiotic infusion and there are different types with different volumes and flow rates. Rapid infusion with a hypodermic syringe has many advantages if used properly19 (Table).
Each unit selects one or another of these systems depending on the characteristics of the center and each patient, taking into consideration whether medication can be prepared at home or in the pharmacy and the particular problems of each case. Antibiotics with the fewest possible daily doses should be used.
Monitoring and Follow Up
Therapy Monitoring
Most of the CF units monitored treatment through appointments and telephone calls, 20% through patient's telephone calls alone, 5% programmed appointments with the patient and in another 5% home visits were made by the nurse.
There are different ways of monitoring effectiveness of treatment ranging from simple telephone contact to more or less frequent home visits by different health care staff depending on the characteristics of each unit:
--Programmed home visits by a specialized or primary care nurse.
--Monitoring at the CF unit a week after treatment has started (when the week's medication is picked up) or at the local hospital if the home is far from the unit.
--Home visit by the doctor only if the nurse has detected problems.
--Telephone contact only. In this case, the information on the benefit or otherwise of the treatment is based on the patient's subjective assessment.
Although the ideal way of monitoring treatment is programmed home visits by the nurse, nowadays this is not feasible for most units. Consequently, a week after initiating therapy, a programmed visit to the CF unit is recommended, in addition to telephone contact. Patients should undergo a thorough revision in hospital on completion of treatment.
Problem Solving During Follow Up
During working hours, problems are solved by the CF unit nurse in 70% of the cases; the other 30% are solved by other hospital departments, including hospital ward and day hospital nurses. After hours, 85% of problems were solved by other hospital departments; the CF nurse was only responsible in 10% of cases.
The problems that occur during HIVAT, then, will be solved by the unit in charge of the treatment or by groups chosen by the unit.
Venous Access Complications
The most common problems with venous access were loss of the access (75% of cases), difficulty with medication infusion (10%) and reddening or inflammation at the point of access (10%).
In agreement with these results, problems with the use of catheters are normally minor, according to reports in the literature.20-22
Staff Responsible for Follow Up
The team or staff responsible for treatment can vary according to the circumstances. Approximately half the participating groups answered that the nurse and doctor of the CF unit were responsible for the monitoring and follow up of HIVAT; 25% said it was the doctor alone; 15%, the nurse alone, and 5%, the nurse from the primary health care center.
Primary health care staff were reported to be little involved, either not knowing how to deal with such a complex illness or because of the CF unit doctor's or the patient's own lack of confidence.
Groups responsible for HIVAT can be a) CF units; b) home health care teams; c) primary health care providers in contact with the CF unit; and d) the patient's local hospital working together with the CF unit or the home health care team.
Home health care teams, in CF centers that have them, are the obvious choice for monitoring and following up therapy as visiting patients at home is how they work. When the patient lives a long way from the CF unit, monitoring and follow up should be programmed with the local hospital or primary health care center, maintaining contact with the CF unit. In Spain, the CF units currently assume all responsibility for follow up and monitoring of patients receiving HIVAT.
Legal Aspects
Informed consent must be given, though only 25% of units receive it. The promotion of its use was agreed upon as it is a legal requisite (the Spanish Ley General de Sanidad 14/1986).
During the discussion of legal aspects, participants agreed on the need to draw up regulations on home health care.
Quality Control
All the specialists present at the conference were in favor of establishing quality controls for HIVAT, although it was not an item on the questionnaire. Quality control is essential in order to obtain reliable evidence that what is being done is giving the predefined results.
First it is necessary to:
--Assess the effectiveness of the program, evaluating the response to the treatment and possible complications.
--Increase the effectiveness of the home health care team, adding other CF treatments to home care (nutrition, oxygen therapy, or mechanical breathing) and to other chronic patients, according to the characteristics of each center.
--Draw up a written working plan.23
Recommendations
--All the CF units that participated in this consensus prescribed HIVAT. The application of this type of therapy may well increase over the next years.
--Despite scarce evidence in the literature, we assume that HIVAT is as efficacious as treatment administered in hospital, given careful selection of patients, individualized therapy and adequate supervision.
--The advantages of HIVAT outweigh the disadvantages, above all because it allows patients to continue their daily life, working or studying; these aspects are more apparent with adult patients.
--A CF HIVAT program work group must contain a nurse who coordinates the program, and a doctor in charge, normally a pneumologist.
--HIVAT must be voluntary and given to patients who fulfill the inclusion criteria and do not have any of the established exclusion criteria.
--Patient and family training for HIVAT is the responsibility of the CF unit nurse or the hospital ward/day hospital nurse or the home health care team nurse, according to the characteristics of the unit. Participation of primary health care nurses will be required for patients living far from the hospital.
--Patient training must be initiated during their first hospital admission; they can be allowed to go home when they or their family feel ready. In subsequent therapies it is enough to give only the first dose in hospital. Patients, particularly children, can be admitted to hospital for 24 to 36 hours in special cases. Training time, it should be remembered, will vary from one individual to another.
--Information on all HIVAT programs must be clear and concise and should be accompanied by an explanatory leaflet or reinforced by audiovisual methods.
--We think a peripheral vein can be used as first option or a peripherally accessed central vein. Conventional central venous catheterization can be used if there is no peripheral access.
--Various devices can be used for the administration of IV antibiotics depending on what is available to each unit. Direct infusion is an option to be considered.
--Although home visits by the nurse are the best option for HIVAT, nowadays this is not feasible for most units. For this reason, a programmed visit to the unit a week after initiating therapy, together with telephone contact, and a thorough revision at the end of treatment, are recommended.
--Monitoring and follow up of HIVAT must be carried out by the unit in charge, which is normally the CF unit.
--Informed consent is needed for HIVAT.
--Quality control over the whole health program is a basic aim, in order to obtain reliable evidence that what is being done is giving the results previously defined.
The organization of the Consensus Conference on HIVAT should pave the way to the designing of an appropriate home health care program, the basic characteristics of which should be defined, although aspects will vary from place to place. Each center must evaluate all aspects and the benefits of a program of this nature should be pressed on administrative and medical directors, as it improves patients' and families' quality of life and saves hospitals a great deal of money that could be used on research and other purposes.
Acknowledgments
Project initiated by Dr Héctor Escobar, president of the Fundación Sira Carrasco para Ayuda a la Fibrosis Quística. Coordination: R. Girón and A. Salcedo.
Participating Spanish Doctors and Hospitals
A. Salcedo (Hospital Infantil Universitario Niño Jesús. Madrid).
R.M. Girón, I. Jiménez and A. Mancha (Hospital de la Princesa. Madrid).
H. Escobar and L. Máiz (Hospital Ramón y Cajal. Madrid).
C. Muñoz and A. Escribano (Hospital Clínico. Valencia).
J. Pérez Frías, E. Pérez Ruiz and C. Olveira (Hospital Carlos Haya. Málaga).
J.M. Marugán (Hospital de León. León).
F. Sánchez-Valverde, J.E. Olivera and E. Aznal (Hospital Virgen del Camino. Navarra).
J. de Gracia and A. Álvarez (Hospital Universitario Vall d'Hebron. Barcelona).
C. Antelo, I. Barrio and M.C. Martínez (Hospital Infantil La Paz. Madrid).
J. Ferrer, J.A. López, I. Cortell, A. Solé and P. Morales (Hospital Universitario La Fe. Valencia).
J. Cuyás (Hospital Dr. Negrín. Las Palmas).
O. Asensio and M. Bosque (Hospital de Sabadell. Barcelona).
C. Vázquez and F. Baranda (Hospital de Cruces. Bilbao).
S. Heredia and A. Marco (Hospital Miguel Servet. Zaragoza).
J.L. Séculi (Hospital de San Joan de Deu. Barcelona).
L. García Alonso and J. Sirvent Gómez (Complexo Hospitalario Juan Canalejo. Hospital Infantil Teresa Herrera. A Coruña).
G. García Hernández, C. Garfia Castillo, J. Manzanares and M.T. Martínez Martínez (Hospital Universitario 12 de Octubre. Madrid).
C. Bousoño, E. Ramos, M. Roza, G. Gea, C. Álvarez and L. Rodrigo (Hospital Central de Asturias. Oviedo).
J.M. Román, J. Figuerola, B. Osona and A. Rosell (Hospital Son Dureta. Palma de Mallorca).
Correspondence: Dr. R.M. Girón Moreno.
Servicio de Neumología. Hospital Universitario de la Princesa.
Diego de León, 62. 28006 Madrid. España.
E-mail: med002861@nacom.es
Manuscript received February 28, 2003.
Accepted for publication May 6, 2003.