Chronic obstructive pulmonary disease (COPD) mortality and morbidity have increased significantly worldwide in recent decades. Although cigarette smoke is still considered the main risk factor for the development of the disease, estimates suggest that between 25% and 33% of COPD patients are non-smokers. Among the factors that may increase the risk of developing COPD, biomass smoke has been proposed as one of the most important, affecting especially women and children in developing countries.
Despite the epidemiological evidence linking exposure to biomass smoke with adverse health effects, the specific cellular and molecular mechanisms by which this pollutant can be harmful for the respiratory and cardiovascular systems remain unclear. In this article we review the main pathogenic mechanisms proposed to date that make biomass smoke one of the major risk factors for COPD.
Las tasas de mortalidad y morbilidad de la enfermedad pulmonar obstructiva crónica (EPOC) han aumentado mundialmente de forma significativa durante las últimas décadas. A pesar de que el humo de tabaco se sigue considerando el principal factor etiopatogénico para el desarrollo de la enfermedad, se estima que entre una tercera y una cuarta parte de los pacientes con EPOC son no fumadores. De todos los factores de riesgo que pueden incrementar la probabilidad de sufrir EPOC en estos sujetos se ha propuesto al humo de biomasa como uno de los más importantes, afectando sobre todo a mujeres y a niños de países emergentes.
Aunque existen numerosas evidencias epidemiológicas que relacionan la exposición al humo de biomasa con efectos nocivos para la salud, todavía no se conocen bien los mecanismos celulares y moleculares específicos mediante los cuales este contaminante puede suponer una noxa para los sistemas respiratorio y cardiovascular. En esta revisión se recogen los mecanismos patogénicos propuestos hasta la fecha que sitúan al humo de biomasa como uno de los principales factores de riesgo para la EPOC.
Chronic obstructive pulmonary disease (COPD) is a slow, progressive process, characterized by permanent, not fully reversible, airflow obstruction in the bronchioles and by destruction of the pulmonary parenchyma, known as emphysema.1 The onset of COPD is associated with chronic exposure to toxic gases and particles, in particular tobacco smoke, that triggers an abnormal inflammatory pulmonary and systemic response.2–5 However, the causes of the disease are multifactorial, and both genetic disorders and environmental factors may be involved.1
Although tobacco smoke is still considered the primary risk factor for developing COPD,6 a growing body of evidence indicates that between a quarter and a third of COPD cases are diagnosed in non-smokers.7 Estimates based on Global Initiative for Lung Disease (GOLD) spirometric criteria suggest that 17%–38.8% of COPD patients worldwide are non-smokers.7 In the United States, the United Kingdom, and Spain, rates of COPD in non-smokers are 23%,8 22.9%,9 and 23.4%,10 respectively. Epidemiological studies conducted in developing countries have also revealed a high prevalence of COPD among non-smokers. In the PLATINO study,11 conducted in five cities in Latin America, 26% of all subjects with irreversible airflow obstruction were non-smokers. However, a study conducted in China using similar methodology found an overall prevalence of COPD of only 5.2% among non-smokers.12
The most important risk factors that may contribute to the development of COPD in these subjects include genetic predisposition, occupational exposure to dust and chemicals, diet, recurrent respiratory infections in childhood, and air pollution, both indoors and outdoors.13 In the latter group, there has been a recent surge of interest in biomass smoke as a significant pollutant in the pathogenesis of COPD.13
Biomass Smoke as a PollutantBiomass smoke as a source of energy is defined as all organic material from animals or vegetable matter that can be used as fuel. This definition covers a wide range of materials, the most common being wood, agricultural residue, such as branches and dried grass, animal dung, and charcoal.14 These materials are generally burnt in poorly-ventilated fireplaces and stoves, and generate substantial amounts of harmful pollutants that can reach exposure levels of between 10 and 20 times those recommended by the World Health Organization (WHO).15
Smoke from burning biomasses contains more than 250 organic compounds that can vary depending on the type of material and combustion conditions.16 A wide range of gaseous pollutants can be generated, including carbon monoxide, ammonia, hydrocyanic acid, formaldehyde, nitrogen oxides and sulfur, and volatile organic compounds, such as benzene and polycyclic aromatic hydrocarbons (PAH), like benzopyrene. The latter two are potent carcinogens in humans.17,18 Moreover, biomass smoke contains a mixture of solid and liquid particles that vary in concentration, size, surface and chemical composition, known as particulate matter (PM).18 Some particles are respirable and are classified according to their aerodynamic diameter as PM10 (coarse particles with an aerodynamic diameter of 10μm or less), PM2.5 (fine particles with an aerodynamic diameter of 2.5μm or less) and PM0.1 (ultrafine particles with an aerodynamic diameter of 0.1μm or less). PM10 particles are generally composed of minerals with crystalline or amorphous components and elements absorbed from diverse sources, such as fungi, bacteria or endotoxins.19 The high transition metal content of PM2.5 particles, which generally consist of a carbon nucleus with surface absorption of organic and inorganic components,20 enhances their oxidative stress-generating potential. Ultrafine PM0.1 particles, meanwhile, with their high PAH content, are also powerful oxidative stress inducers.20,21
Many studies support the hypothesis that inhalation of these pollutants is harmful to the health and constitutes a risk factor not only for COPD, but also for respiratory infections, asthma, lung cancer, cardiovascular disorders, cataracts, cerebrovascular accidents or even adverse effects on neonatal development.22
Risk PopulationAt present, around 50% of the world's population and 90% of homes in rural areas burn biomass as their main source of energy in the home, for cooking or heating.23 Over 80% of homes in China, India and sub-Saharan Africa use biomass for fuel, while in rural areas in Latin America, the proportion ranges between 30% and 70%.24 Although the use of biomass fuel is particularly extensive in developing countries, the rising cost of fossil fuel, such as petroleum products or natural gas, along with environmental concerns about CO2 emissions, have also led to an increase in the use of biomass as fuel in developed countries, such as Germany, Finland, Canada, Australia or the United States.24–27
Around 3 billion people worldwide are exposed to biomass smoke, compared to 1 billion tobacco smokers, suggesting that biomass smoke may be a more significant risk factor for developing COPD than tobacco smoke on a worldwide basis.14 Moreover, for sociocultural reasons, women and children form the main population groups exposed to this pollutant, which is thought to contribute to the death of 2 million women and children globally every year.28 This is because in many developing countries, it is common for women and children to remain at home for long periods, cooking or in close proximity to stoves.
In these settings, women are thought to spend on average over 60000h of their life cooking on a biomass stove, during which time they inhale over 25 million liters of polluted air.23 These data are significant, since it has been shown that respiratory symptoms and airflow limitation increase in line with exposure to biomass smoke.29,30 Consequently, several studies have shown that women who use biomass as fuel for cooking have a higher prevalence of respiratory symptoms of COPD than those who do not use this type of fuel.31–39 Indeed, 50% of deaths due to COPD in developing countries are attributable to biomass smoke, and 75% of these are in women.40
Although COPD mainly affects adults, several studies have shown that many chronic diseases may originate in the development of the fetus and the early years of life.41 In a recent study, Epstein et al.42 found the children of mothers using biomass in their homes had a lower birth weight than those whose of mothers using other fuel sources. These results are relevant, since low birth weight, being associated with defective lung development and function during childhood and adulthood, is known to be an independent risk factor for the development of COPD.43 Another study performed in a rural area in India found that forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) were significantly lower in schoolchildren from homes burning biomass fuel than in those from homes using liquefied petroleum gas.44 Biomass smoke in indoor environments also increases the risk of respiratory infections.45–47 This factor should also be taken into account, since infections in childhood can predispose an individual to developing COPD in adulthood.24
Although women and children are most exposed to biomass smoke, a recent study in 922 COPD patients in 7 cities in Asia found that 31% of men had also been exposed to this pollutant.48 Although the percentage is lower than in women (44.8%), the figure is significant. The results of a meta-analysis in Asian and non-Asian subjects revealed that individuals exposed to biomass smoke have a 2.44-fold greater risk of developing COPD than the unexposed population.49 Exposure was identified as a risk factor in both men (OR: 4.30; 95% CI: 1.85–10.01) and women (OR: 2.73; 95% CI: 2.28–3.28).49 Data from another recent study confirm the association between airflow obstruction in non-smokers and cooking with charcoal in both sexes.50
Pathogenic Mechanisms of Biomass Smoke in Chronic Obstructive Pulmonary DiseaseBiomass smoke contains many compounds similar to those in tobacco smoke,51 therefore, COPD caused by both types of smoke might be expected to present similar clinical and radiological characteristics.52,53 However, some differences are observed between COPD from biomass smoke and COPD from tobacco smoke53–60 (Table 1). From a histopathological point of view, for example, patients with biomass COPD have more bronchiolitis, more anthracosis and pulmonary fibrosis, and more airway wall thickening than smokers with COPD, who present with more pulmonary emphysema.55,57–59 A recent paper published by Krimmer et al.61 showed that fibroblasts exposed in vitro to biomass smoke increased their production of fibronectin.
Characteristics Differentiating Chronic Obstructive Pulmonary Disease (COPD) Caused by Tobacco Smoke and by Biomass Smoke.
| Characteristic | Biomass smoke COPD | Tobacco smoke COPD | Reference |
|---|---|---|---|
| Reduced DLCO | + | +++ | 54 |
| Oxygen saturation at rest and during exercise | + | +++ | 55 |
| Bronchial hyperreactivity on methacoline challenge | +++ | + | 56 |
| Goblet-form cell hyperplasia | + | +++ | 57 |
| Emphysema | + | +++ | 55–60 |
| Airway wall thickening | +++ | + | 57, 58 |
| Anthracosis | +++ | + | 57 |
| Pulmonary artery intimal hyperplasia | +++ | + | 57 |
COPD: chronic obstructive pulmonary disease; DLCO: diffusion lung capacity for carbon monoxide.
Despite epidemiological evidence relating biomass smoke with COPD, little is known about the cell and molecular mechanisms that cause exposure to this type of pollutant to be harmful to health. It has been recently suggested that biomass smoke may contribute to the onset of COPD by fostering a pulmonary and systemic inflammatory state, and by increasing the genotoxic effect of oxidative stress, among other mechanisms of cell damage (Figs. 1 and 2).
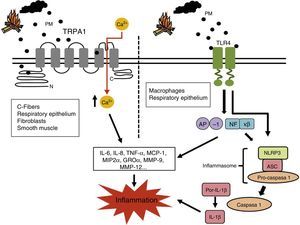
Pro-inflammatory mechanisms of biomass smoke. PM-stimulated cells generate numerous inflammatory mediators, including IL-6, IL-8, TNF-α, MCP-1, MIP2α and GROα. These can generate a second wave of mediators that include enzymes, such as MMP-9 and MMP-12, involved in tissue remodeling typical of COPD. It has been suggested that TRP ion channels in various lung cells can be activated by PMs in biomass smoke. This would lead to increased cytoplasmic Ca2+ and an intracellular signaling cascade that would increase the production of these pro-inflammatory mediators. The biological material in biomass smoke PMs (e.g., endotoxin) may also activate pro-inflammatory transcription factors AP-1 y NF-κβ via signaling initiated in the TLR receptors. These receptors and agents causing intracellular damage, such as ROS, can also activate inflammasome NLRP3, thereby constituting another possible pro-inflammatory pathway triggered by biomass smoke.
AP-1: activating protein-1; GROα: growth-related oncogen α; ROS: reactive oxygen species; IL-6: interleukin-6; IL-8: interleukin-8; MCP-1: monocyte chemoattractant protein 1; MIP2α: macrophage inflammatory protein 2α; MMP-9: matrix metalloproteinase 9; MMP-12: matrix metalloproteinase 12; NF-κβ: nuclear factor kappa-light-chain-enhancer of activated B cells; PMs: respirable particulate matter; TLR4: Toll-like receptor 4; TNF-α: Tumor necrosis factor-α; TRP1: Transient Potential Receptor 1 ion channel.
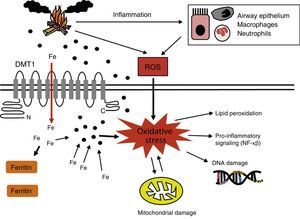
Biomass smoke oxidative mechanisms and genotoxicity. Oxidative stress generated by biomass smoke ROS can cause direct damage to cell macromolecules, such as proteins or lipids, and can act as a genotoxic factor in producing DNA lesions. Mitochondrial membrane potential can also be affected by oxidative damage, resulting in increased ROS production in these organelles. Some authors61 have proposed that PMs sequester iron from the lung cells, affecting iron homeostasis. When cells detect iron deficiency, they try to reestablish availability of this metal by generating ROS to decrease it and promoting the expression of iron importers such as DMT1. As a result of the increased intracellular iron levels, the amount of ferritin increases. However, these oxidizing molecules are known to activate pro-inflammatory transcription factors, such as NF-κβ. Finally, inflammatory cells activated by biomass smoke are another source of ROS.
DMT1: divalent metal transporter 1; NF-κβ: nuclear factor kappa-light-chain-enhancer of activated B cells; ROS: reactive oxygen species.
Abundant evidence is available to demonstrate that exposure to biomass smoke causes lung inflammation. Women exposed to biomass smoke have higher alveolar levels of neutrophils, eosinophils, monocytes, mastocytes, lymphocytes and macrophages, as well as higher sputum levels of interleukin (IL)-6, IL-8 and tumor necrosis factor (TNF)-α than those who use other types of fuel.62–64 Gene expression of some of these pro-inflammatory mediators, such as IL-8, TNF-α, matrix metalloproteinase (MMP)-9 and MMP-12, increases in parallel with exposure to biomass smoke, as recently demonstrated by Guarnieri et al.,65 while another study shows that this pollutant alters the expression of genes associated with CD8+ T-cell activation.66 The pro-inflammatory effects of biomass smoke are not restricted to the lung, since increases have been reported in CD8+ T-cells, natural killer (NK) cells, IL-6, IL-8, TNF-α, C-reactive protein (CRP) and monocyte chemoattractant protein (MCP)-1 in the blood of exposed individuals.67–69 Studies performed in samples of induced sputum, bronchoalveolar lavage (BAL) fluid and blood show that COPD patients exposed to biomass smoke have higher levels of inflammatory cells (neutrophils and eosinophils), IL-8, CRP and MMP-12, and increased MMP-9 activity, compared to control subjects.23,70,71
It has been suggested that biomass smoke may induce an inflammatory response via the Transient Potential Receptor (TRP) ion channels of the lung cells72 (Fig. 1). These channels can be activated by a wide range of stimuli, including PM, meaning that the cells are responsive to changes in their environment. When a TRP channel is activated, it mediates Na+ and Ca2+ flow across the plasma membrane to the cytoplasma, stimulating other voltage-dependent channels and influencing cell events, such as transcription, transduction, contraction and migration.73,74 The TRP channel superfamily is divided into seven subfamilies, of which TRPA1, V1, V4 and M8 receptors have shown capacity for regulating inflammatory responses.72 TRPA1 receptors, found in airway C-fibers, bronchiolar epithelial cells, fibroblasts and smooth muscle cells, may induce IL-6 and IL-8 production and increase bronchiolar macrophage inflammatory protein (MIP) 2α and growth-related oncogene (GRO)α cytosine expression after exposure to biomass smoke.74–78
Another pro-inflammatory mechanism proposed for biomass smoke is the activation of toll-like receptors (TLR) on airway macrophages and epithelial cells, which would in turn activate the nuclear factor kappa-light-chain-enhancers of activated B cells (NF-κβ) and activator protein-1 (AP-1) activity20 (Fig. 1). According to this hypothesis, these pathways may be activated by the small amounts of biological material (e.g., endotoxin, fungal spores, etc.) present in the PM. Becker et al.79 showed that IL-6 and IL-8 production in alveolar macrophages and epithelial cells exposed to PM could be inhibited by TLR4 and TLR2 antagonists or blocking antibodies. In another study, TLR4 and TLR2-deficient mouse macrophages produced less TNF-α and IL-6 after exposure to PM.80
In another recent study, Hirota et al.81 put forward inflammasome NLRP3, a protein complex that can initiate an inflammatory response to agents causing cell damage, as another key molecular mechanism in the PM-induced inflammatory response (Fig. 1). These authors reported in vivo and in vitro evidence of increased IL-1β production and neutrophilia in airways when this molecular complex was activated after exposure to PM.
Kido et al.82 determined the link between pulmonary and systemic inflammation and biomass smoke. In another study in mice, they showed that pro-inflammatory mediators, such as IL-6, pass from the lungs to the bloodstream after exposure to PM10.
Oxidative Mechanisms and GenotoxicitySeveral studies conducted in sputum and blood samples of women and children chronically exposed to biomass smoke showed an increase in the production of reactive oxygen species (ROS) and a decrease in antioxidant mechanisms such as superoxide dismutase (SOD), glutathione (GSH) and ascorbic acid.63,64,69,83–85 Other studies reported an increase in lipid peroxidation products, such as malondialdehyde (MDA) in the blood of subjects exposed to biomass smoke,85–88 and a correlation between the levels of this compound and reduced VEF1/CVF.83 Increases in oxidative stress markers in DNA in sputum samples89 and peripheral blood mononuclear cells (PBMC)87,90 have also been described in subjects chronically exposed to biomass smoke. Diminished capacity to repair cell damage has also been reported in these subjects.62,85,90 These results are supported by several studies in animal models91–94 or in vitro cell lines89,95–104 that found increased production of ROS, cytokines, lipid peroxidation products and oxidative DNA damage or impaired antioxidant mechanisms after exposure to biomass smoke. Oxidative stress, then, appears have a significant role in activating the harmful effects of this pollutant.
ROS and reactive nitrogen species (RNS) may contribute to the pathogenesis of COPD and other lung diseases, such as asthma or bronchiectasis105–107 by oxidizing proteins, lipids, carbohydrates and DNA.108 Some of the components of biomass smoke, such as PM, can induce oxidative stress via several mechanisms: directly, by the ROS-generating effect of the physicochemical characteristics of the particles or the effect of soluble components (e.g., organic compounds or transition metals), or indirectly, by activation of inflammatory cells that can lead to generation of ROS or RNS.20,64,96
Some authors have suggested that one of the most significant oxidative mechanisms of biomass smoke is the disturbance of iron homeostasis in lung cells72 (Fig. 2). When PMs become lodged in the lower respiratory tract, they sequester iron from the tissues, diminishing the amount of iron available to the cells. Attempts by the cells to reestablish the availability of the metal lead to increased iron importation and reduction through the Fenton reaction (Fe2++H2O2→Fe3++•OH+OH−), generating ROS in the process.72,109,110 ROS derived from these reactions are also known to activate transcription factors, such as NF-κβ, increasing the production of pro-inflammatory mediators.111 Moreover, damage caused by mitochondrial oxidation may affect the membrane potential of these organelles, thereby increasing ROS production112 (Fig. 2).
This hypothesis is supported by evidence of increased numbers of iron-laden macrophages (siderophages) in the sputum of women exposed to biomass smoke,63 and increased expression of divalent metal transporter-1 (DMT1, one of the main iron importers) and ferritin levels in epithelial cells exposed to this pollutant.72
ConclusionsBiomass may be a source of renewable and sustainable energy, but its combustion is one of the major sources of pollution, both indoors and outdoors. Abundant epidemiological, clinical and experimental evidence has shown that exposure to biomass smoke is harmful to health and predisposes individuals to a range of diseases, including COPD.
The pathogenic mechanisms considered to date include increased pulmonary and systemic inflammation, and promotion of an oxidative stress state that may cause macromolecular cell damage, including DNA changes.
Efforts must be made to increase awareness of the risk of exposure to biomass smoke. Significant data are available on the benefits of reducing exposure this pollutant by replacing biomass with biogas or by improving combustion systems and ventilation of stoves and kitchens.113–116 A report on the HUMAN platform initiative117 found that the construction of kitchens to reduce smoke pollution in the homes of risk populations is feasible and inexpensive. Promoting the use of cleaner energy sources, such as electric, by way of incentives, particularly in more deprived populations in developing countries during the winter months, may also be an effective measure.
AuthorshipDr Rafael Silva and Dr Miguel Oyarzún made a critical review of the manuscript. Dr Jordi Olloquequi conceived and produced the paper.
Conflict of interestsThe authors state that they have no conflict of interests.
The authors thank Ms Carmen Gloria Muñoz Pincheira for her administrative support in the generation of this review article.
Please cite this article as: Silva R, Oyarzún M, Olloquequi J. Mecanismos patogénicos en la enfermedad pulmonar obstructiva crónica causada por exposición a humo de biomasa. Arch Bronconeumol. 2015;51:285-292.