Cystic fibrosis (CF) is a disease produced by a defect in the transmembrane conductance regulator protein, CFTR. Currently, the morbidity and mortality associated with CF are fundamentally related with the lung affectation that is a consequence of this defect. With the progression of the disease, there is an increase in the isolation of non-fermenting gram-negative bacilli colonizing these patients.
The genus Pandoraea arises from a reclassification of species included within the “Burkholderia cepacia complex”. It is made up of 9 species susceptible only to tetracycline, imipenem and cotrimoxazole.
We report the first clinical case in Spain of colonization by Pandoraea sputorum in a patient diagnosed with CF at the age of eleven. After several previous colonizations by different Pseudomonas species in September 2005, a gram-negative bacillus was isolated in sputum, which was identified by sequencing and mass spectrometry (MALDITOF-MS) as P. sputorum, only sensitive to piperacillin-tazobactam, cotrimoxazole and imipenem. From 2005 to 2008, chronic colonization by this microorganism was associated with deterioration in lung function that was recuperated after treatment with piperacillin-tazobactam and imipenem. In 2010, this microorganism was once again isolated and treated with imipenem, to which the patient responded favorably.
Currently, it is not known whether this microorganism is a chronic colonizer, whether it produces a transitory infection or it constitutes an important problem in CF patients, but given its special characteristics of sensitivity to anti-microbial drugs, the correct identification of this genus is essential. Mass spectrometry seems to be a valid technique that is faster than sequencing methods for identifying these species.
La fibrosis quística (FQ) es una enfermedad producida por un defecto de la proteína reguladora de la conductancia transmembrana CFTR. En la actualidad la morbimortalidad asociada a FQ está relacionada fundamentalmente con la afectación pulmonar consecuencia de este defecto. Con el progreso de la enfermedad se incrementa el aislamiento de bacilos gramnegativos no fermentadores como colonizadores en estos pacientes.
El género Pandoraea surge de una reclasificación de especies integradas en el «complejo Burkholderia cepacia», y comprende 9 especies sensibles solamente a tetraciclinas, imipenem y cotrimoxazol.
Describimos el primer caso clínico, en España, de colonización por Pandoraea sputorum en una paciente diagnosticada de FQ a los 11 años que, tras varias colonizaciones previas por diferentes especies de Pseudomonas, en septiembre de 2005 se aisló en esputo un bacilo gramnegativo identificado por secuenciación y espectrometría de masas (MALDITOF-MS) como P. sputorum, resultando solo sensible a piperacilina-tazobactam, cotrimoxazol e imipenem. Desde 2005 a 2008 se demostró la colonización crónica por este microorganismo, asociándose a un deterioro de la función pulmonar que se recuperó tras tratamiento con piperacilina-tazobactam e imipenem. En 2010 se volvió a aislar este microorganismo y se trató con imipenem, fármaco al que la paciente respondió favorablemente.
En la actualidad se desconoce si este microorganismo es un colonizador crónico, produce una infección transitoria o constituye un problema importante en el paciente con FQ, pero dadas las características especiales de sensibilidad a antimicrobianos, la identificación correcta de este género es fundamental. La espectrometría de masas parece ser una técnica válida y más rápida que los métodos de secuenciación para la identificación de estas especies.
Cystic fibrosis (CF) is a recessive autosomal disease produced by a congenital defect in the long arm of chromosome 7 that codes for the cystic fibrosis transmembrane conductance regulator protein, CFTR, which is expressed in the respiratory system, pancreas, bile ducts, sweat glands and the genital-urinary system. As a consequence of this alteration, there is a dysfunction in the exocrine glands, pancreatic failure and obstructions of the airways resulting from alterations in mucociliary transport and the increased production and viscosity of the mucus. Due to improvements in the management of pancreatic alterations, the majority of the morbidity and mortality associated with CF are related with the lung affectation; between 80% and 90% of patients die due to respiratory failure, repetitive infections and an exaggerated inflammatory response.1 The quality of life and survival of the these patients increase as the number of annual exacerbations and the bacterial load in the respiratory infections decrease, This makes it essential to control the colonization and the evaluation of the deterioration of the respiratory function for the management of CF patients.2–4 Among the isolated colonizing microorganisms in these patients are Pseudomonas aeruginosa and Staphylococcus aureus, and, as a consequence of the antimicrobial treatment and lung deterioration, there is increased isolation of non-fermenting Gram-negative bacilli Stenotrophomonas maltophilia, Acromobacter spp. and Burkholderia cepacia. It is important to define and know the sensitivity patterns of the microorganisms that colonize the airways in this type of patients and especially the clinical aspects related with the colonization by multi-resistant pathogens as well as the specific therapeutic options for each situation. It is thus evident that microbiological studies are essential for a correct control and follow-up of CF patients.3,5–7
The genus Pandoraea, made up of non-fermenting and non-sporulating motile Gram-negative bacilli, emerges as the result of a re-study of the species of the “Burkholderia cepacia complex”, Ralstonia picketii and Ralstonia paucula,8,9 and was described by Coenye et al. in the year 2000. It has 9 species, only 5 of which are named: P. apista, P. pulmonicola, P. pnomenusa, P. sputorum and P. norimbergensis. The identification at the species level of this new genus is complex as they often present phenotypic patterns similar to other bacteria species; therefore, it is necessary to use PCR methods based on 16S rRNA.6,10 The antibiotic therapy for treating the infections caused by species of the genus Pandoraea is complex due to the limited number of antibiotics to which they are sensitive: tetracycline, imipenem and co-trimoxazole.11,12
In an attempt at better understanding the clinical implications of this potential pathogen in CF patients, we report the clinical case of a patient diagnosed with CF with bronchiectasis and several exacerbations secondary to infection by Pandoraea sputorum and Pseudomonas aeruginosa.
Clinical NotesThe patient is a woman diagnosed with CF at the age of 11 (1992), with heterozygous genotype delta F508. In 1994, she was hospitalized for the first time in the pediatrics unit due to exacerbation. Pseudomonas aeruginosa was isolated in sputum and then treated with ceftazidime 1g/8h plus tobramycin 160mg/12h for 20 days. She was maintained with inhaled tobramycin until 2002, when microbiological controls revealed Pseudomonas putida, and treatment was begun with nebulized amikacin 250mg/12h. In 2004, coinciding with the transfer of the patient to the pulmonology department, the amikacin was withdrawn and nebulized tobramycin was prescribed at a dosage of 300mg/12h in 28-day on/off cycles. The patient presented bronchospasm and hyperactivity demonstrated by spirometry; therefore the change was made to sodium colistimethate 2MU/12h, which was well-tolerated. In March 2004, the patient had an episode of exacerbation with sputum culture positive for Pseudomonas putida, and meropenem was added to the usual treatment at a dosage of 500mg/8h for 20 days.
In September 2005, a non-fermenting Gram-negative bacillus was isolated in sputum, which was later identified as Pandoraea sputorum. The sputum was spread in a standard culture medium and in 24h there were mucoid colonies growing, oxidase positive, that were analyzed with the Vitek-2 automated system and API 20NE (bioMérieux, France), obtaining inconclusive results with both methods. The definitive identification was done with 16S ribosomal RNA sequencing at the ISCIII national reference center. Parallely, in our laboratory it was identified using the MALDITOF-MS system (Shimadzu Corporation), which correctly classified it at the species level with a confidence percentage of 90%. The antibiogram was done with the E-test method (AB bioMérieux, France), and was only sensitive to piperacillin-tazobactam (MIC=16μg/ml), co-trimoxazole (MIC=1μg/ml) and imipenem (MIC=1.5μg/ml) and resistant to ceftazidime (MIC>256μg/ml), cefepime (MIC>16μg/ml), aztreonam (MIC>16μg/ml), meropenem (MIC>8μg/ml), tobramycin (MIC>256μg/ml), amikacin (MIC>256μg/ml) and colistin (MIC>4μg/ml). In microbiological studies done from September 2005 to September 2008, chronic colonization by this microorganism was seen, but no antimicrobial treatment was begun.
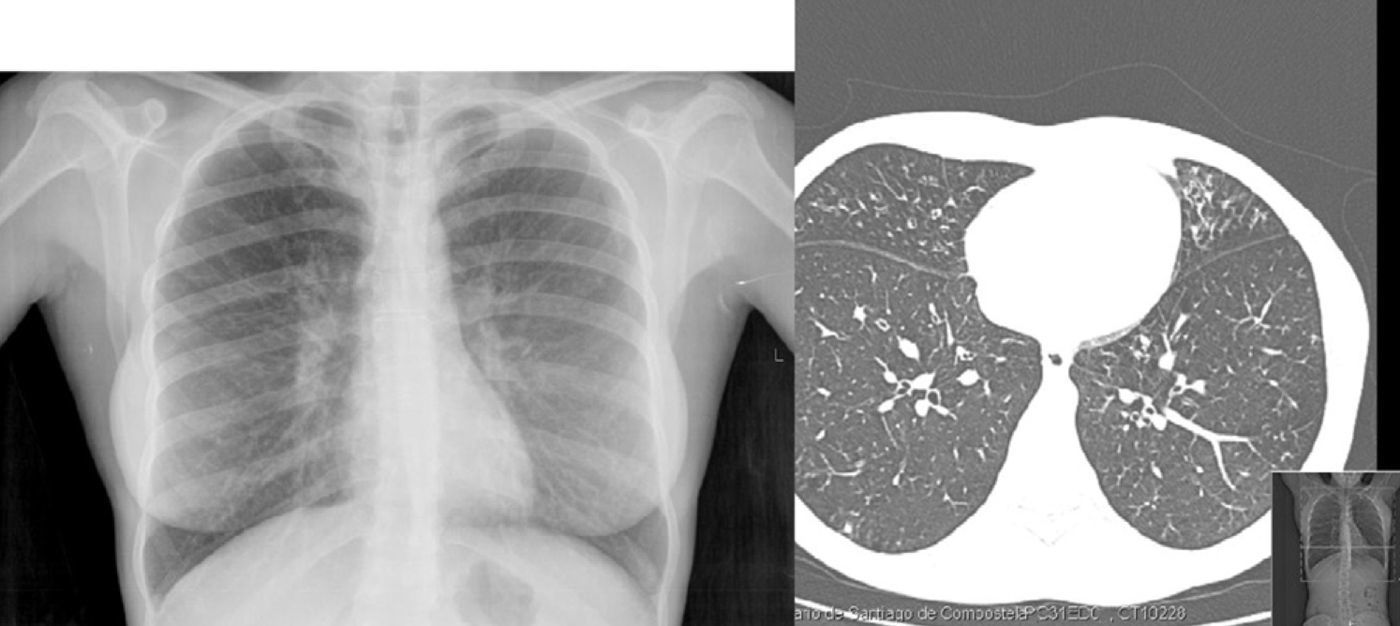
In October 2008, the patient presented low-grade fever and greater dyspnea, with oxygen saturation on pulse-oximetry at 92% while breathing room air, and a significant deterioration of the lung function (FEV1: 50%). Imaging studies revealed bronchiectasis in the middle lobe and lingula (Fig. 1). Given the persistence of P. sputorum in the sputum culture, treatment was initiated with piperacillin-tazobactam at 100mg/8h for 15 days. Afterwards, the patient was symptom-free for 2 years, with an increase in lung function reaching 65%. In April 2010, the patient presented bloody expectoration and medium-exertion dyspnea, and in respiratory samples P. sputorum was once again isolated with the same antibiotic sensitivity profile. Treatment was initiated with imipenem 500mg/8h for 20 days, and from the start the patient evolved favorably and the microbiological control cultures were negative. In a later sputum culture (May 2010), Citrobacter freundii sensitive to co-trimoxazole was isolated; therefore, the patient received this treatment at a dosage of 100mg/6h, and improvement was seen from a functional and clinical standpoint.
In the respiratory samples from September 2010, P. sputorum was once again isolated.
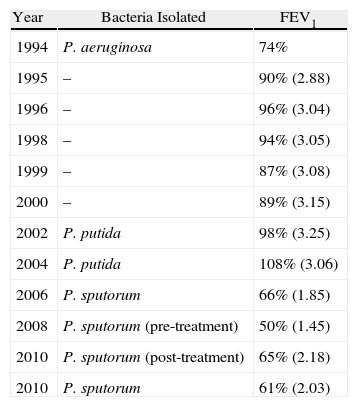
Table 1 shows the functional evolution of the patient along with the microorganisms that were isolated.
Functional Evolution of the Patient. FEV1: Forced Expiratory Volume in 1s.
| Year | Bacteria Isolated | FEV1 |
| 1994 | P. aeruginosa | 74% |
| 1995 | – | 90% (2.88) |
| 1996 | – | 96% (3.04) |
| 1998 | – | 94% (3.05) |
| 1999 | – | 87% (3.08) |
| 2000 | – | 89% (3.15) |
| 2002 | P. putida | 98% (3.25) |
| 2004 | P. putida | 108% (3.06) |
| 2006 | P. sputorum | 66% (1.85) |
| 2008 | P. sputorum (pre-treatment) | 50% (1.45) |
| 2010 | P. sputorum (post-treatment) | 65% (2.18) |
| 2010 | P. sputorum | 61% (2.03) |
There is currently little data about the results and the clinical course of the patients colonized by species of the genus Pandoraea. It is unknown whether in CF patients this microorganism behaves as a chronic colonizer like Pseudomonas aeruginosa,13 produces a transitory infection like Klebsiella spp., Enterobacter spp. and Citrobacter sp., or constitutes an important problem like that associated with Burkholderia cepacia, B. anocepacea or B. multivorans.14 The clinical evidence of the invasive potential of this genus has been demonstrated in previous cases reporting colonization by Pandoraea sp. in patients with CF,14–17 those reporting bacteriemia by this microorganism11,18 and in another 4 cases of patients without CF in whom the isolation of this microorganism coincided with a significant deterioration in lung function.19 However, we still have very little knowledge about the virulence factors and the mechanism of pathogenicity of this genus or of its role in the lung damage in CF. In addition, the Pandoraea spp. strain has been found in a great variety of clinical samples: blood, sputum, urine, samples from the upper respiratory tract and lung tissue.12
Recent studies of patients without previous pathology have allocated greater virulence to the species P. pulmonicola when compared to P. apista and P. pnomenusa. It is necessary, however, to carry out more studies with larger numbers of patients and include chronic pathologies to be able to reach definitive conclusions. In the same manner, such studies should be complimented with analyses of possible competition between species, as in many cases there is an observed co-colonization by other pathogens.12
In our patient, chronic colonization by P. sputorum led to a clear decline in lung function, probably due to the production of IL-6 and IL-8 as demonstrated in previous studies.9 It has also been observed that, in patients colonized by this microorganism, anti-Pandoraea antibodies may develop, which would contribute to the pathogenic potential of this microorganism.18
In our review of the literature, we have only found one previous case of colonization by P. sputorum in a lung transplant recipient, and ours is the first case described in Spain in a CF patient. However, in the medical literature there are cases of patients with CF and recipients of lung transplants in whom the isolation of other bacteria of the genus Pandoraea (P. pulmicola and P. apista) was associated with a severe deterioration in lung function and even with multiorgan failure and death.17,18
One of the most interesting aspects of the microbiological follow-up of the patients with cystic fibrosis lies in the demonstration of clonality of the different strains over time, which may show evidence of chronic colonization patterns or sporadic situations related with clinical symptoms.5 Recent studies suggest, although with no real evidence, a patient-to-patient transmission,12 and some centers even indicate that these patients be isolated in spite of there being neither consensus nor any specific recommendations.12,14,20
Given the special characteristics of sensitivity to antimicrobial agents of this genus, which can be considered multi-resistant microorganisms, and the varying clinical evolution of patients with CF, the correct identification of the non-fermenting Gram-negative bacilli is fundamental. Currently, the species of this new genus are often incorrectly identified as other phenotypically similar bacteria, and they are especially confused with species of the Burkholderia cepacia complex. In the case of the species of the genus Pandoraea, their identification implicated the need for sequencing methods that were only within the means of reference laboratories. Today, mass spectrometry, a technique that has been recently introduced in microbiology laboratories, is an important contribution to the identification of these species.
On the other hand, colonization by multi-resistant pathogens that are difficult to identify with conventional methods highlights the fact that the microbiology of CF is becoming more and more complex. It is therefore necessary to establish reference laboratories in order to minimize to the fullest extent the errors in identification of these new emerging pathogens, as well as possible new phenotypes in the future.7
Please cite this article as: Martínez-Lamas L, et al. Colonización por Pandoraea sputorum en un paciente con fibrosis quística. Arch Bronconeumol. 2011;47:571-4.