Non-tuberculous mycobacteria (NTM) isolates are becoming more common. The main objective of our study was to establish the number and diversity of NTM species in our region and their distribution according to the source sample, age and gender of the patients, and to analyze clinically significant isolates.
MethodologyProspective study of all NTM isolated in Asturias from 2005 to 2012. Samples were processed following internationally accepted guidelines. Statistical analysis was based on Fisher's exact test for 2×2 contingency tables.
ResultsA total of 3284 mycobacteria were isolated: 1499 Mycobacterium tuberculosis complex (MTB) and 1785 NTM. During the study, NTM isolation rates increased while MTB isolation decreased. NTM were more frequent in men (p<0.001). M. gordonae was the most frequently isolated species but did not cause disease in any case. NTM isolates from 212 patients were associated with clinically significant disease (17.1%). M. kansasii and M. avium were most commonly associated with disease. The number of M. kansasii isolates from men was statistically significant (p<0.01).
ConclusionsIn our study, NTM isolates increased by 35%, compared with a 21% decline in cases of MTB. Both isolation of NTM and clinically significant cases were more common in men. Only 17.1% of NTM isolates were associated with disease, most commonly M. avium complex and M. kansasii.
Los aislamientos de micobacterias no tuberculosas (MNT) son cada vez más frecuentes. El objetivo principal de nuestro estudio fue conocer el número y la variedad de especies de MNT en nuestra región, su distribución según el origen de la muestra, y la edad y sexo de los pacientes; asimismo, analizar pormenorizadamente los aislamientos clínicamente significativos.
MetodologíaEstudio prospectivo que incluye todas las MNT aisladas en Asturias durante el período 2005–2012. Las muestras se procesaron siguiendo directrices internacionalmente aceptadas. Para el tratamiento estadístico de los datos se utilizaron tablas de contingencia 2×2 aplicando el test exacto de Fisher.
ResultadosSe aislaron 3.284 micobacterias: 1.499 Mycobacterium tuberculosis complex (MTB) y 1.785 MNT. A lo largo del estudio se incrementaron los aislamientos de MNT y se redujeron los de MTB. Los aislamientos de MNT fueron más numerosos en hombres que en mujeres (P<0,001). M. gordonae, la especie más frecuentemente aislada, no originó enfermedad en ningún caso. El aislamiento fue clínicamente significativo en 212 pacientes (17,1%), siendo M. kansasii y M. avium las especies que más frecuentemente causaron enfermedad. La diferencia de aislamientos de M. kansasii entre mujeres y hombres fue estadísticamente significativa (p<0,01).
ConclusionesEn nuestro estudio, los aislamientos de MNT se incrementaron un 35%, frente a un descenso del 21% de los casos de MTB. Tanto los aislamientos de MNT como los casos clínicamente significativos fueron más frecuentes en hombres. Solo un 17,1% de las MNT aisladas, principalmente M. avium complex (MAC) y M. kansasii, ocasionaron enfermedad.
Non-tuberculous mycobacteria (NTM) have gained prominence in recent years, and now account for between 30% and 50% of the total number of mycobacteria isolated in microbiology laboratories1; this phenomenon may be associated with an increased incidence of disease caused by NTM. The reasons for this increase are not entirely clear. The introduction of automated liquid culture systems in mycobacteria laboratories in the 1990s may have played a decisive role in improving diagnostic yield,2–4 although in their first decade of continued use no such dramatic increase in NTM isolation rates was reported.5–15 In general, data on the incidence and prevalence of NTM are scant2 and probably determined by the ability of each laboratory to isolate these organisms and the available diagnostic tools.
More than 170 species of mycobacteria have been described to date (http://www.bacterio.cict.fr/m/mycobacterium.html).
The most frequently isolated species are Mycobacterium avium complex (MAC), M. gordonae, M. kansasii, M. marinum, M. xenopi, M. fortuitum, M. chelonae and M. abscessus.
Generally, most isolates are not clinically significant, but a recent study in the United States showed that the number of deaths from disease due to NTM was increasing.5 It is important to assess the significance of the isolates by analyzing their specific clinical context in line with the recommendations of internationally recognized scientific societies, such as the American Thoracic Society (ATS), the British Thoracic Society (BTS) and the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR).16–18
Some diseases are often listed among the risk factors for mycobacterial infection: chronic obstructive pulmonary disease (COPD), pneumoconiosis, bronchiectasis, previous tuberculosis, post-radiotherapy fibrosis, chronic pulmonary aspiration (esophageal disease), cystic fibrosis, immune deficiency, HIV infection, alcoholism, cancer (lung or other sites), and diabetes mellitus. However, it should be noted that a high percentage of patients present no risk factors.2,5,16
The aim of this study was to determine the number and diversity of NTM species isolated in our region and their distribution according to the source of the sample, and the age and gender of the patient. Clinically significant isolates were also analyzed in detail.
MethodologyThis was a prospective study that included all mycobacteria isolated in Asturias (Spain) during the period 2005–2012. Isolates came from the 8 public hospitals in the region (mean population of 1,079,626 inhabitants) that systematically submit the isolated strains to the Regional Mycobacteria Reference Unit for identification and/or sensitivity testing.
Protocols following internationally accepted guidelines (American Society for Microbiology [ASM])19 were used for the initial processing of the clinical samples.
The commercially available preparation BBL MycoPrep® (Specimen Digestion/Decontamination Kit, Becton-Dickinson) was used for the pretreatment digestion-decontamination phase.
Solid culture media (Löwenstein-Jensen) and liquid media with automated reading systems (BACTEC™ MGIT™ 960 [Mycobacterial Growth Indicator Tube] and/or Bact/ALERT® MP), were used for culture, according to the methodology of each hospital.
Species level identification was carried out using off-the-shelf techniques combining PCR and reverse hybridization (INNO-LiPA® Mycobacteria V2 and GENOTYPE® Mycobacterium CM/AS) and proprietary techniques (PRA20 16S-23S rRNA21) combining PCR-RFLP. Sequencing was not required in any of the clinically significant cases.
The criteria listed in the ATS/IDSA 2007 guidelines16 were used to define a case as clinically significant. For statistical analysis of the data, 2×2 contingency tables were used to verify the independence of dichotomous variables, applying the Fisher's exact test. A value of P<0.05 was considered statistically significant.
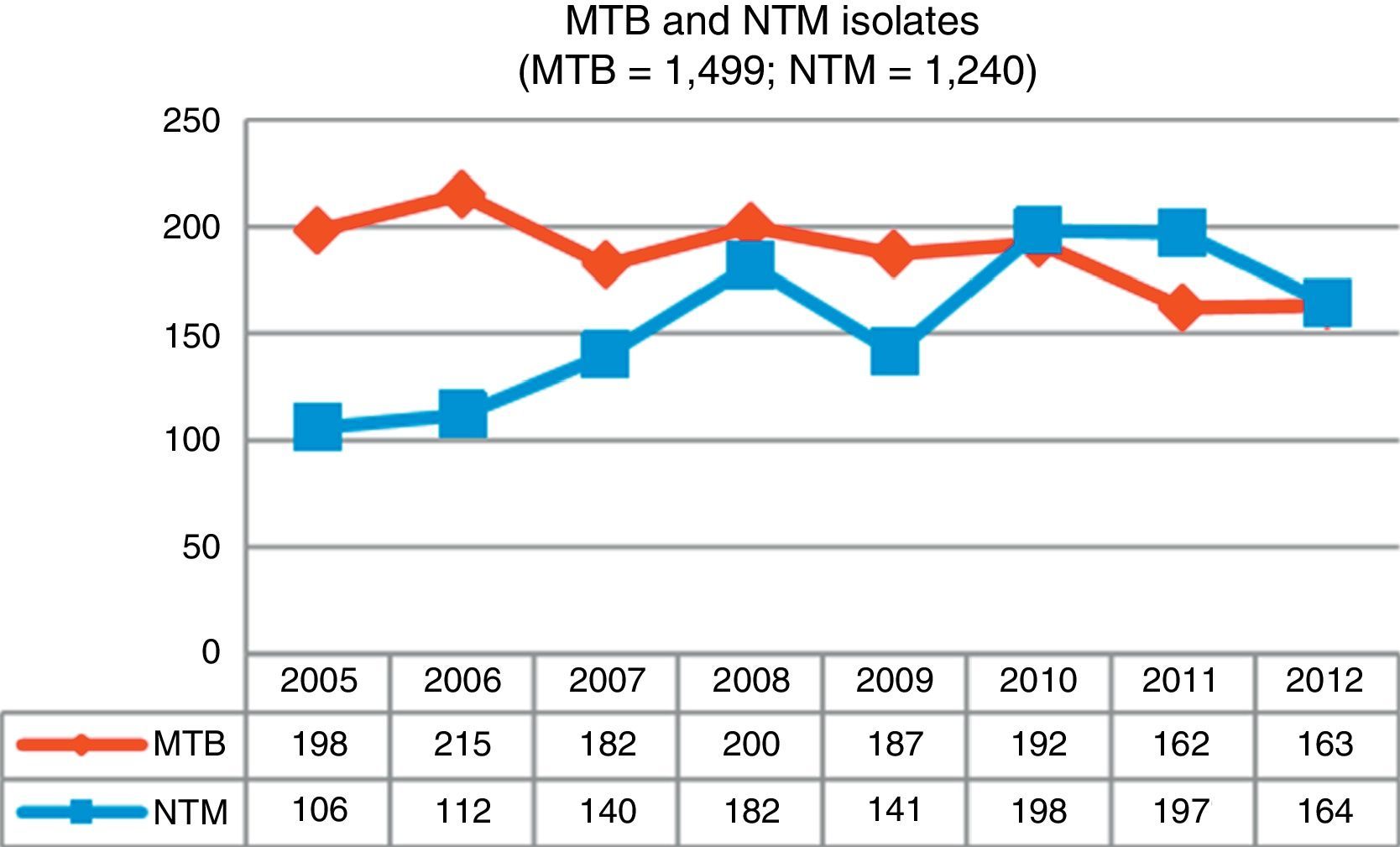
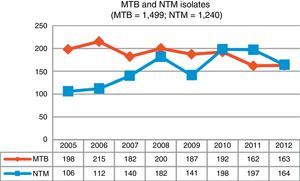
ResultsDuring the study period, 3,284 mycobacteria isolated from 37,041 clinical samples were identified: 1,499 (45.7%) were M. tuberculosis complex (MTB) and 1,785 (54.3%) were NTM. Fig. 1 shows changes in the number of isolates by mycobacteria groups over the 8-year period of analysis; a growing trend for NTM and a waning trend for MTB can be observed.
In total, 1,785 NTM isolates were obtained from samples from 1,240 patients; 898 were men (72.4%) and 342 were women (27.6%). Mean patient age was 65 years (66.6 for men and 63.6 for women).
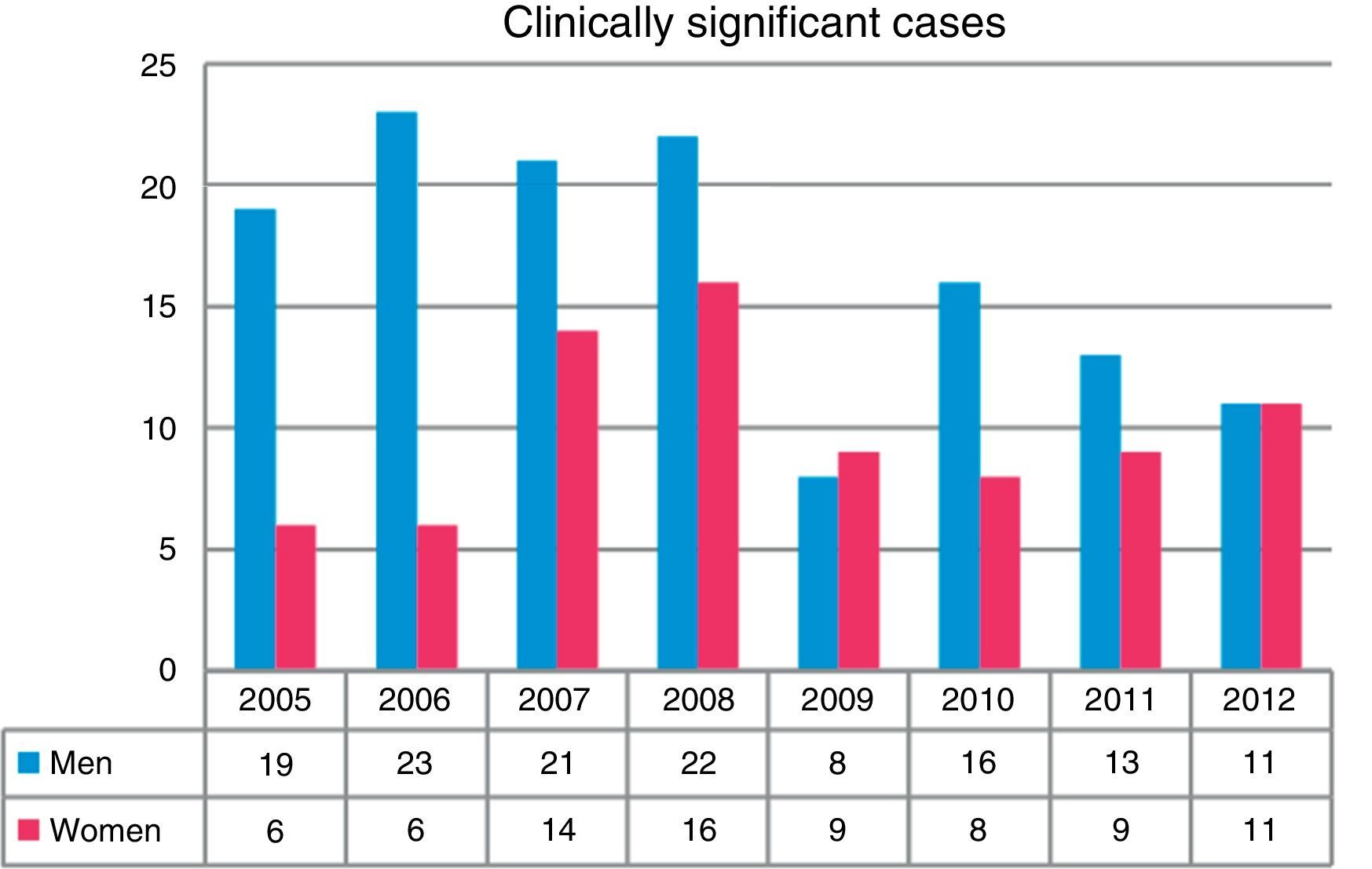
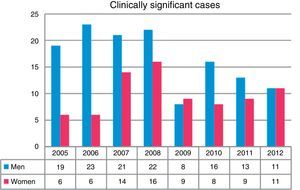
Overall, more isolates were obtained from men than from women (P<0.001). A total of 43.5% of the isolates were obtained in the first 4 years of the study (2005–2008). The greatest differences in isolation rates between men and women were recorded in the same period, while in the next 4-year period (2009–2012) the differences leveled out, until the last year when rates became equal (Fig. 2). NTM isolates from 212 patients were associated with clinically significant disease (17.1% of all patients in whom NTM were isolated), while the others were classified as colonization.
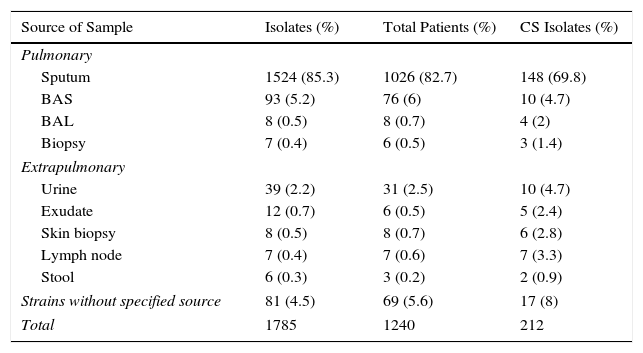
NTM isolates were obtained mainly from the respiratory tract (91.4%), 4% from various extrapulmonary sites, and in 4.5% of the cases the strains were sent to our laboratory without specifying the origin of the clinical sample (Table 1).
NTM Isolates According to Source of Sample.
| Source of Sample | Isolates (%) | Total Patients (%) | CS Isolates (%) |
|---|---|---|---|
| Pulmonary | |||
| Sputum | 1524 (85.3) | 1026 (82.7) | 148 (69.8) |
| BAS | 93 (5.2) | 76 (6) | 10 (4.7) |
| BAL | 8 (0.5) | 8 (0.7) | 4 (2) |
| Biopsy | 7 (0.4) | 6 (0.5) | 3 (1.4) |
| Extrapulmonary | |||
| Urine | 39 (2.2) | 31 (2.5) | 10 (4.7) |
| Exudate | 12 (0.7) | 6 (0.5) | 5 (2.4) |
| Skin biopsy | 8 (0.5) | 8 (0.7) | 6 (2.8) |
| Lymph node | 7 (0.4) | 7 (0.6) | 7 (3.3) |
| Stool | 6 (0.3) | 3 (0.2) | 2 (0.9) |
| Strains without specified source | 81 (4.5) | 69 (5.6) | 17 (8) |
| Total | 1785 | 1240 | 212 |
BAL: bronchoalveolar lavage; BAS: bronchial aspirate; CS: clinically significant.
Sputum was the most common respiratory sample, followed by bronchial aspirate (BAS), bronchoalveolar lavage (BAL), and lung biopsy (Table 1). Clinically significant NTM in respiratory samples were more frequently isolated from lung biopsies (42.8%) and BAL (50%). The lowest percentage was recorded in sputum (9.5%).
Most isolates obtained from extrapulmonary samples were from urine, followed by exudate/abscess, skin biopsy, lymph node biopsy, and stool. NTM isolates in extrapulmonary samples were clinically significant in 100% of lymph node biopsies, 75% of skin biopsies, 41.6% of exudate/abscesses, 33.3% of stool samples, and 25.6% of urine samples.
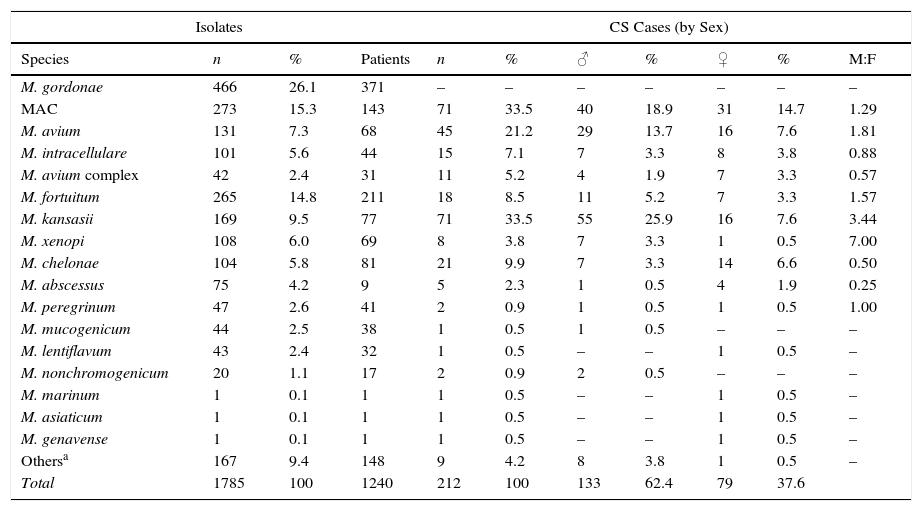
The most frequently isolated species were M. gordonae (26.1%), MAC (15.3%), M. fortuitum (14.8%), M. kansasii (9.5%), M. xenopi (6.0%), M. chelonae (5.8%) and M. abscessus (4.2%). Overall, these accounted for 81.8% of the total number of isolates. Table 2 shows the rates of isolation of the different species of NTM per patient, and their distribution by sex, in clinically significant cases. M. gordonae was the most frequently isolated species in our setting, but did not cause disease in any case. M. kansasii and M. avium were the two species of NTM most commonly associated with disease (33.5 and 21.2% of cases, respectively). Differences were found between the species isolated from men and from women. Among men, M. kansasii (25.9%), M. avium (13.7%), M. fortuitum (5.2%), M. xenopi (3.3%) and M. chelonae (3.3%) were the most frequent. In women, the most common species were M. avium (7.5%), M. kansasii (7.5%), M. chelonae (6.5%), M. fortuitum (3.3%) and M. abscessus (1.9%). In the case of M. kansasii, the difference in the number of isolates between women and men was statistically significant (P<0.01).
Isolates Identified by Species, Patient Numbers and Sex Distribution in Clinically Significant Cases.
| Isolates | CS Cases (by Sex) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | n | % | Patients | n | % | ♂ | % | ♀ | % | M:F |
| M. gordonae | 466 | 26.1 | 371 | – | – | – | – | – | – | – |
| MAC | 273 | 15.3 | 143 | 71 | 33.5 | 40 | 18.9 | 31 | 14.7 | 1.29 |
| M. avium | 131 | 7.3 | 68 | 45 | 21.2 | 29 | 13.7 | 16 | 7.6 | 1.81 |
| M. intracellulare | 101 | 5.6 | 44 | 15 | 7.1 | 7 | 3.3 | 8 | 3.8 | 0.88 |
| M. avium complex | 42 | 2.4 | 31 | 11 | 5.2 | 4 | 1.9 | 7 | 3.3 | 0.57 |
| M. fortuitum | 265 | 14.8 | 211 | 18 | 8.5 | 11 | 5.2 | 7 | 3.3 | 1.57 |
| M. kansasii | 169 | 9.5 | 77 | 71 | 33.5 | 55 | 25.9 | 16 | 7.6 | 3.44 |
| M. xenopi | 108 | 6.0 | 69 | 8 | 3.8 | 7 | 3.3 | 1 | 0.5 | 7.00 |
| M. chelonae | 104 | 5.8 | 81 | 21 | 9.9 | 7 | 3.3 | 14 | 6.6 | 0.50 |
| M. abscessus | 75 | 4.2 | 9 | 5 | 2.3 | 1 | 0.5 | 4 | 1.9 | 0.25 |
| M. peregrinum | 47 | 2.6 | 41 | 2 | 0.9 | 1 | 0.5 | 1 | 0.5 | 1.00 |
| M. mucogenicum | 44 | 2.5 | 38 | 1 | 0.5 | 1 | 0.5 | – | – | – |
| M. lentiflavum | 43 | 2.4 | 32 | 1 | 0.5 | – | – | 1 | 0.5 | – |
| M. nonchromogenicum | 20 | 1.1 | 17 | 2 | 0.9 | 2 | 0.5 | – | – | – |
| M. marinum | 1 | 0.1 | 1 | 1 | 0.5 | – | – | 1 | 0.5 | – |
| M. asiaticum | 1 | 0.1 | 1 | 1 | 0.5 | – | – | 1 | 0.5 | – |
| M. genavense | 1 | 0.1 | 1 | 1 | 0.5 | – | – | 1 | 0.5 | – |
| Othersa | 167 | 9.4 | 148 | 9 | 4.2 | 8 | 3.8 | 1 | 0.5 | – |
| Total | 1785 | 100 | 1240 | 212 | 100 | 133 | 62.4 | 79 | 37.6 | |
CS: clinically significant.
“Others” includes species that could not be identified at the species level (61), isolation of 2 species simultaneously (31), and identified species occurring at a very low frequency (75), which in no case were considered pathogenic: M. smegmatis (9), M. simiae (7), M. terrae (7), M. celatum (7), M. goodii (6), M. chitae (5), M. malmoense (4), M. neoaurum (3), M. scrofulaceum (3), M. triviale (3), M. szulgai (3), M. interjectum (3), M. confluentis (2), M. flavescens (2), M. phlei (2), M. thermoresistibile (2), M. immunogenum (1), M. gadium (1), M. gilvum (1), M. heckeshornense (1), M. parafortuitum (1), M. parascrofuleaceum (1) and M. pulveris (1).
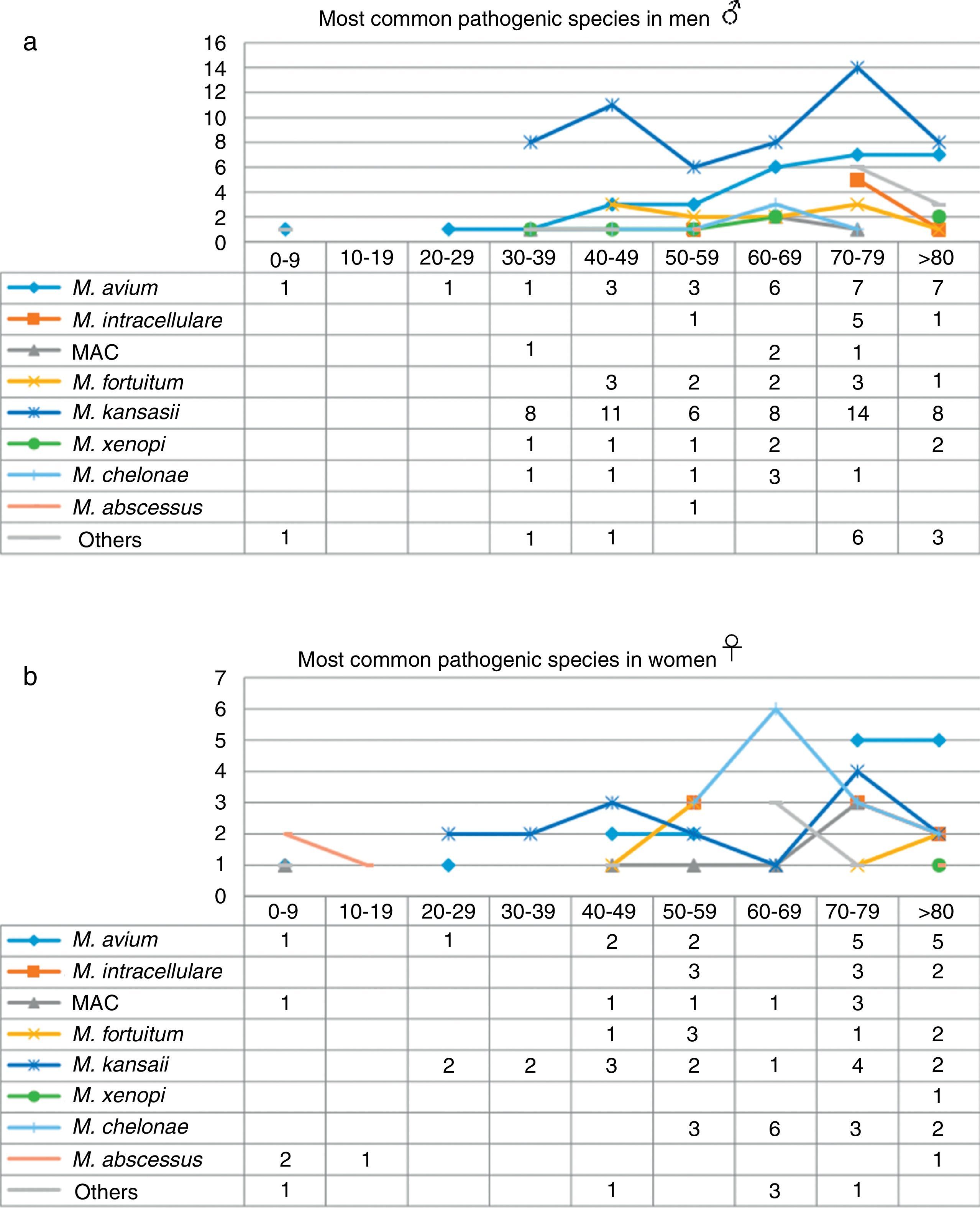
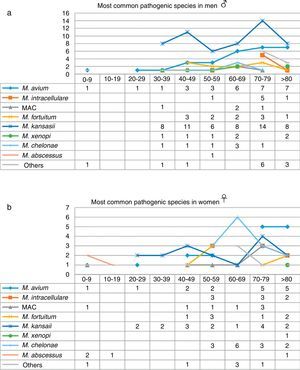
When the data were analyzed by sex and age range (Fig. 3), the largest number of NTM isolates occurred in patients older than 70 years of age, mainly men, while the incidence in younger patients was higher in women, particularly under the age of 30 (P=0.01). With regard to the correlation between the species isolated and the age range, we found that M. kansasii, M. avium, MAC and M. abscessus were isolated in younger women, while in men in that age range, only M. avium was isolated. M. intracellulare, in contrast, was only isolated in women over 50 years of age, reaching maximum values in patients older than 70 years. The largest number of isolates of M. intracellulare and MAC occurred in men over the age of 60 years. In the case of M. xenopi, all isolates, with the exception of 1, were obtained from men. In contrast, twice as many M. chelonae isolates were obtained from women than from men.
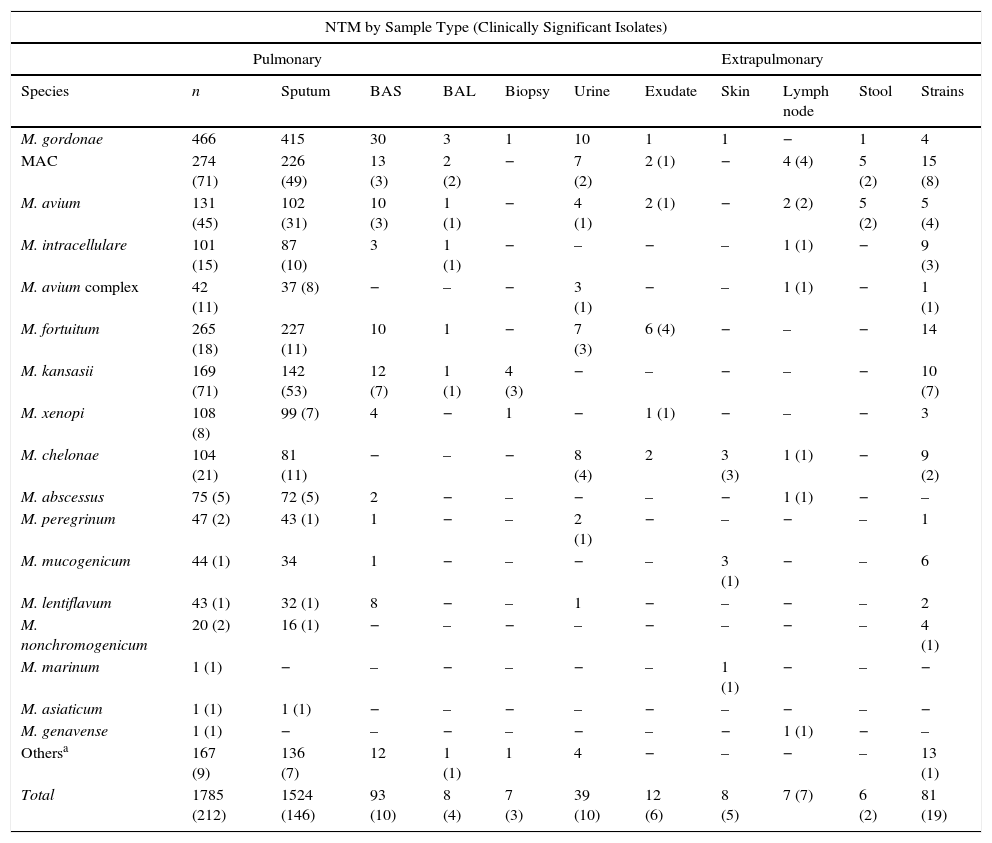
With regard to the source of the sample (Table 3), practically all NTM were isolated from lung samples (91.42%), mostly in sputum, except for M. marinum and M. genavense, isolated from a skin biopsy and a lymph node biopsy, respectively. In patients younger than 20 years, mycobacteria were isolated mainly from extrapulmonary sites.
NTM Species by Sample Type.
| NTM by Sample Type (Clinically Significant Isolates) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pulmonary | Extrapulmonary | ||||||||||
| Species | n | Sputum | BAS | BAL | Biopsy | Urine | Exudate | Skin | Lymph node | Stool | Strains |
| M. gordonae | 466 | 415 | 30 | 3 | 1 | 10 | 1 | 1 | − | 1 | 4 |
| MAC | 274 (71) | 226 (49) | 13 (3) | 2 (2) | − | 7 (2) | 2 (1) | − | 4 (4) | 5 (2) | 15 (8) |
| M. avium | 131 (45) | 102 (31) | 10 (3) | 1 (1) | − | 4 (1) | 2 (1) | − | 2 (2) | 5 (2) | 5 (4) |
| M. intracellulare | 101 (15) | 87 (10) | 3 | 1 (1) | − | – | − | – | 1 (1) | − | 9 (3) |
| M. avium complex | 42 (11) | 37 (8) | − | – | − | 3 (1) | − | – | 1 (1) | − | 1 (1) |
| M. fortuitum | 265 (18) | 227 (11) | 10 | 1 | − | 7 (3) | 6 (4) | − | – | − | 14 |
| M. kansasii | 169 (71) | 142 (53) | 12 (7) | 1 (1) | 4 (3) | − | – | − | – | − | 10 (7) |
| M. xenopi | 108 (8) | 99 (7) | 4 | − | 1 | − | 1 (1) | − | – | − | 3 |
| M. chelonae | 104 (21) | 81 (11) | − | – | − | 8 (4) | 2 | 3 (3) | 1 (1) | − | 9 (2) |
| M. abscessus | 75 (5) | 72 (5) | 2 | − | – | − | – | − | 1 (1) | − | – |
| M. peregrinum | 47 (2) | 43 (1) | 1 | − | – | 2 (1) | − | – | − | – | 1 |
| M. mucogenicum | 44 (1) | 34 | 1 | − | – | − | – | 3 (1) | − | – | 6 |
| M. lentiflavum | 43 (1) | 32 (1) | 8 | − | – | 1 | − | – | − | – | 2 |
| M. nonchromogenicum | 20 (2) | 16 (1) | − | – | − | – | − | – | − | – | 4 (1) |
| M. marinum | 1 (1) | − | – | − | – | − | – | 1 (1) | − | – | − |
| M. asiaticum | 1 (1) | 1 (1) | − | – | − | – | − | – | − | – | − |
| M. genavense | 1 (1) | − | – | − | – | − | – | − | 1 (1) | − | – |
| Othersa | 167 (9) | 136 (7) | 12 | 1 (1) | 1 | 4 | − | – | − | – | 13 (1) |
| Total | 1785 (212) | 1524 (146) | 93 (10) | 8 (4) | 7 (3) | 39 (10) | 12 (6) | 8 (5) | 7 (7) | 6 (2) | 81 (19) |
BAL: bronchoalveolar lavage; BA: bronchial aspirate; CS: clinically significant.
“Others” includes species that could not be identified at the species level (61), isolation of 2 species simultaneously (31), and identified species occurring at a very low frequency (75), which in no case were considered pathogenic: M. smegmatis (9), M. simiae (7), M. terrae (7), M. celatum (7), M. goodii (6), M. chitae (5), M. malmoense (4), M. neoaurum (3), M. scrofulaceum (3), M. triviale (3), M. szulgai (3), M. interjectum (3), M. confluentis (2), M. flavescens (2), M. phlei (2), M. thermoresistibile (2), M. immunogenum (1), M. gadium (1), M. gilvum (1), M. heckeshornense (1), M. parafortuitum (1), M. parascrofuleaceum (1) and M. pulveris (1).
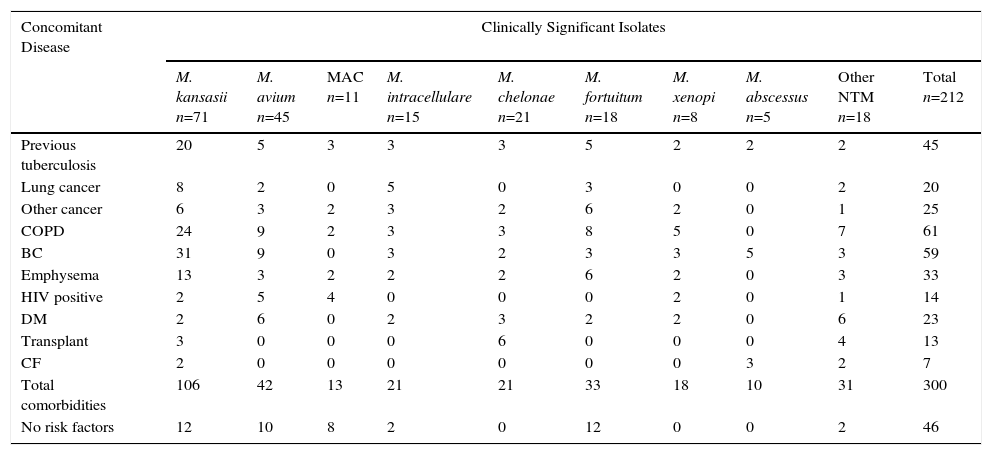
Risk factors and comorbidities among the 212 patients with clinically significant NTM isolates, classified by NTM species, are shown in Table 4. Interestingly, 166 patients (78.3%) had comorbidities, frequently with 2 or more risk factors. With the exception of M. abscessus, a species frequently associated with cystic fibrosis/bronchiectasis, no association was found between any specific underlying disease and the type of NTM identified.
Comorbidity Reported in 212 Patients With Clinically Significant Isolates of NTM.
| Concomitant Disease | Clinically Significant Isolates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M. kansasii n=71 | M. avium n=45 | MAC n=11 | M. intracellulare n=15 | M. chelonae n=21 | M. fortuitum n=18 | M. xenopi n=8 | M. abscessus n=5 | Other NTM n=18 | Total n=212 | |
| Previous tuberculosis | 20 | 5 | 3 | 3 | 3 | 5 | 2 | 2 | 2 | 45 |
| Lung cancer | 8 | 2 | 0 | 5 | 0 | 3 | 0 | 0 | 2 | 20 |
| Other cancer | 6 | 3 | 2 | 3 | 2 | 6 | 2 | 0 | 1 | 25 |
| COPD | 24 | 9 | 2 | 3 | 3 | 8 | 5 | 0 | 7 | 61 |
| BC | 31 | 9 | 0 | 3 | 2 | 3 | 3 | 5 | 3 | 59 |
| Emphysema | 13 | 3 | 2 | 2 | 2 | 6 | 2 | 0 | 3 | 33 |
| HIV positive | 2 | 5 | 4 | 0 | 0 | 0 | 2 | 0 | 1 | 14 |
| DM | 2 | 6 | 0 | 2 | 3 | 2 | 2 | 0 | 6 | 23 |
| Transplant | 3 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 4 | 13 |
| CF | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 7 |
| Total comorbidities | 106 | 42 | 13 | 21 | 21 | 33 | 18 | 10 | 31 | 300 |
| No risk factors | 12 | 10 | 8 | 2 | 0 | 12 | 0 | 0 | 2 | 46 |
BC: bronchiectasis; CF: cystic fibrosis; COPD: chronic obstructive pulmonary disease; DM, diabetes mellitus; HIV: human immunodeficiency virus.
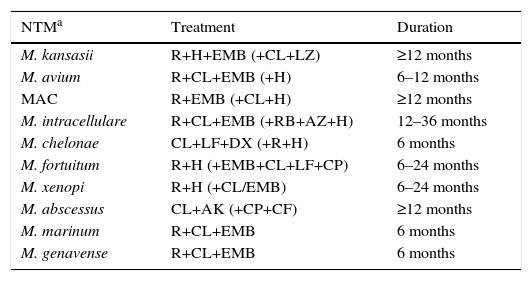
In most cases, treatment was initiated in line with the recommendations published in the standard clinical guidelines (Table 5). Mean treatment duration was 12 months (range 6–36 months). Three percent of patients did not receive treatment.
Treatment Regimens Administered to Patients With Mycobacterial Infection.
| NTMa | Treatment | Duration |
|---|---|---|
| M. kansasii | R+H+EMB (+CL+LZ) | ≥12 months |
| M. avium | R+CL+EMB (+H) | 6–12 months |
| MAC | R+EMB (+CL+H) | ≥12 months |
| M. intracellulare | R+CL+EMB (+RB+AZ+H) | 12–36 months |
| M. chelonae | CL+LF+DX (+R+H) | 6 months |
| M. fortuitum | R+H (+EMB+CL+LF+CP) | 6–24 months |
| M. xenopi | R+H (+CL/EMB) | 6–24 months |
| M. abscessus | CL+AK (+CP+CF) | ≥12 months |
| M. marinum | R+CL+EMB | 6 months |
| M. genavense | R+CL+EMB | 6 months |
AK: amikacin; AZ: azithromycin; CF: cefoxitin; CL: clarithromycin; CP: ciprofloxacin; DX: doxycycline; EMB: ethambutol; H: isoniazid; LF: levofloxacin; R: rifampicin; RB: rifabutin.
NTM with clinically significant isolates not listed in the table (16 cases); 6 were not treated, and of the remaining 10: M. asiaticum (1), M. lentiflavum (1), M. mucogenicum (1), M. peregrinum (2), M. immunogenum (1), M. malmoense (1), M. szulgai (2), M. interjectum (1), although the treatment regimen depended on the species, the most frequent combination being quinolone+macrolide with/without ethambutol and/or rifampin.
With regard to clinical progress, 50 patients (23.6%) died (43 men and 7 women; mean age, 73 years), primarily due to causes associated with their underlying pathology. It is also noteworthy that despite antimycobacterial treatment, NTM was persistently isolated in 5.1% of patients.
DiscussionOver the 8-year study period in Asturias, we observed a 35% increase in NTM isolates compared to a 21% reduction in MTB cases over the same period. Similar results have been reported in other recent studies.7,9,11,12
This same trend has been observed in most industrialized countries, although the incidence rates vary widely depending on the country concerned: 4.8–5.6 cases per 100,000 inhabitants between 2007 and 2012, respectively, in Oregon6; 2.06–2.71 cases per 100,000 inhabitants in Scotland between 2000 and 20107; rates in England, Wales and Northern Ireland rose from 0.9 to 2.9 isolates per 100,000 inhabitants between 1995 and 2006,9 and in Queensland (Australia), rates increased from 2.2 (1999) to 3.2 (2005) cases per 100,000 inhabitants.10
The increase in Asturias has been gradual, rising from a total of 106 isolates at the beginning of our study to 164 isolates obtained in the last year. With regard to isolation rates, 9.82 isolates per 100,000 inhabitants were recorded in 2005, a figure that in 2012 rose to 15.19. Clinically significant cases fell slightly, from 2.31 cases per 100,000 in 2005 to 2.03 in 2012, with a maximum number of 3.52 cases recorded in 2008. The mean isolation rate in the 8-year study period was 2.46 episodes per 100,000 inhabitants; very similar rates were obtained in Scotland during the same period7 and in New York,8 but the numbers recorded in Oregon were slightly higher.6
With regard to the gender distribution of the NTM isolates, three times as many isolates were obtained in men than women. The differences were more marked during the early years of the study, but tended to level out over time; other authors have also made the same observation.7,10,12 Similar findings were reported in clinically significant cases. However, series have also been published in which more NTM were isolated from women.13
With regard to age, the highest number of isolates (approximately 80%) were obtained from the clinical samples of individuals older than 50 years, and, in line with data from other recent studies, greater numbers of species were isolated from patients over 70 years of age.7,9,13–15
The most frequently isolated species of NTM was M. gordonae, the second most frequently isolated mycobacteria worldwide,11 but in none of the cases was it considered pathogenic. The MAC complex, which is the most frequently isolated NTM on a worldwide basis, came in second (15.29%). In another study performed in Spain between 1976 and 1996,22 high percentages of MAC were also obtained in practically all regions except the Canary Islands, although the impact of HIV disease at that time must be taken into account. In Asturias, M. fortuitum and M. kansasii were ranked third and fourth, respectively, while on a global level they are ranked fourth and sixth. In Europe, Slovakia, Poland and the United Kingdom11 recorded the highest rates of M. kansasii isolates. With regard to geographical distribution, in Spain,22M. kansasii has been described mainly in the southern regions, particularly the Community of Valencia, and also in northern regions, such as the Basque Country, where a study performed in Bilbao in 200523 revealed high rates of isolates of this species, while in the border provinces, isolates of this species were virtually non-existent.
With regard to the isolate/pathogenicity ratio found in our series, MAC and M. kansasii were the species that most often caused disease, accounting together for 67% of all cases (142 patients). M. kansasii was predominant in men, the difference being statistically significant (P<0.01). Cases caused by M. xenopi and M. abscessus were similar to those of other studies.7,12M. marinum, M. asiaticum and M. genavense were all isolated once only, and all 3 were considered clinically significant.
Comorbidities were identified in 166 patients (78.3%), most commonly in cases caused by M. xenopi and M. abscessus; in contrast, no risk factors were identified in 46 patients (21.7%), similar to findings published in New York,8 while M. kansasii was the NTM most frequently isolated in cases without comorbidities.24 In transplant patients, M. chelonae and M. kansasii were the most common NTM, unlike other studies,5 in which the most common were MAC and M. abscessus.
With respect to treatment, 97% of patients received a 3-drug combination, and the mean treatment duration was 12 months. NTM persisted in 5.1% of patients, despite antimycobacterial treatment. As this study pools patients diagnosed in 8 different hospitals, treatment regimens were often heterogeneous, particularly with regard to treatment duration and inclusion or exclusion of certain drugs recommended in the literature.25
In conclusion, we can confirm that our region has also seen a marked increase in the number of NTM isolates. Both isolation of NTM and clinically significant cases were more common in men. Only 17.1% of NTM isolates caused disease, the most frequent in these cases being MAC and M. kansasii.
Finally, it should be noted that, paradoxically, despite the increase in the number of NTM isolates registered in our region, the number of cases in which the isolate was considered clinically significant did not increase, but rather fell in the later years of our study, mainly due to the increase of NTM isolates with low pathogenic potential.
AuthorshipJuan José Palacios Gutiérrez, head of the HUCA Regional Mycobacteria Reference Unit directed the study and was responsible for the final review and correction of the manuscript.
Susana Martínez González was responsible for data collection, analysis and interpretation, and preparation of the manuscript.
Arantza Cano Cortés and Luis Alfonso Sota Yoldi participated in the compilation of clinical data and in the critical review of the article.
José María García García acted as a study consultant and participated in the critical review of the article.
Luz María Alba Álvarez was responsible for the literature review.
The SESPA Microbiology Laboratory Network was responsible for isolation of strains submitted by the HUCA Regional Mycobacteria Reference Unit.
Conflict of InterestsThe authors state that they have no conflict of interests.
We thank Ángela Menéndez González, Ángeles Díaz Escalada, Macarena Álvarez Fernández, M.J. Rodríguez and Zulima Velasco for their help and support at all times in the performing the technical aspects of this work, without which we could not have completed this project.
La Red de Laboratorios de Microbiología del SESPA incluye los Servicios de Microbiología de: Hospital de Jarrio, Hospital Carmen y Severo Ochoa, Hospital San Agustín, Hospital de Cabueñes, Hospital de Jove, Hospital Grande Covián, Hospital Álvarez-Buylla, Hospital Valle del Nalón y Hospital Universitario Central de Asturias (HUCA).
Please cite this article as: Martínez González S, Cano Cortés A, Sota Yoldi LA, García García JM, Alba Álvarez LM, Palacios Gutiérrez JJ, et al. Micobacterias no tuberculosas. ¿Una amenaza emergente? Arch Broncopneumol. 2017;53:554–560.