To compare the application of non-invasive ventilation (NIV) versus continuous positive airway pressure (CPAP) in the treatment of patients with cardiogenic pulmonary edema (CPE) admitted to an intensive care unit (ICU).
MethodsIn a prospective, randomized, controlled study performed in an ICU, patients with CPE were assigned to NIV (n=56) or CPAP (n=54). Primary outcome was intubation rate. Secondary outcomes included duration of ventilation, length of ICU and hospital stay, improvement of gas exchange, complications, ICU and hospital mortality, and 28-day mortality. The outcomes were analyzed in hypercapnic patients (PaCO2>45mmHg) with no underlying chronic lung disease.
ResultsBoth devices led to similar clinical and gas exchange improvement; however, in the first 60min of treatment a higher PaO2/FiO2 ratio was observed in the NIV group (205±112 in NIV vs. 150±84 in CPAP, P=.02). The rate of intubation was similar in both groups (9% in NIV vs. 9% in CPAP, P=1.0). There were no differences in duration of ventilation, ICU and length of hospital stay. There were no significant differences in ICU, hospital and 28-d mortality between groups. In the hypercapnic group, there were no differences between NIV and CPAP.
ConclusionsEither NIV or CPAP are recommended in patients with CPE in the ICU. Outcomes in the hypercapnic group with no chronic lung disease were similar using NIV or CPAP.
Comparar la efectividad de la ventilación no invasiva (VNI) frente a la presión positiva continúa en la vía aèc)rea (CPAP) en pacientes ingresados por edema agudo de pulmón (EAP) cardiogèc)nico en una unidad de cuidados intensivos (UCI).
Mèc)todosEnsayo clínico donde 56 pacientes fueron asignados a VNI y 54 pacientes a CPAP. El objetivo primario fue la tasa de intubación. Los objetivos secundarios fueron: duración de ventilación, estancia en UCI y en el hospital, mejoría gasomèc)trica, complicaciones y mortalidad en UCI, hospitalaria y a los 28 días. Los objetivos fueron analizados en pacientes hipercápnicos (PaCO2 >45mmHg) sin patologia pulmonar.
ResultadosAmbos dispositivos obtuvieron similar mejoría clínica y del intercambio gaseoso, sin embargo, la VNI mostró un aumento más rápido de la oxigenación (medido por el cociente PaO2/FiO2) en los primeros 60 minutos de aplicación (205±112 en VNI vs. 150±84 en CPAP, p= 0,02). La tasa de intubación fue similar en ambos grupos (9% en VNI vs. 9% en CPAP, p= 1,0). No hubo diferencias en la duración de la ventilación, ni en la estancia en UCI ni hospitalaria. Tampoco hubo diferencias significativas en la mortalidad en UCI, hospitalaria y a los 28 días entre ambos grupos. En el subgrupo de pacientes hipercápnicos tampoco se observaron diferencias significativas en los objetivos analizados.
ConclusionesLa VNI como la CPAP se pueden emplear en pacientes con EAP en la UCI. En pacientes hipercápnicos sin patología pulmonar no se observa beneficio de la VNI sobre la CPAP.
Cardiogenic pulmonary edema (CPE) is a cause of hypoxemic acute respiratory failure (ARF) due to acute heart failure. Traditionally, the standard medical treatment for CPE has been morphine, nitroglycerin, oxygen therapy and diuretics, and endotracheal intubation.1
Development of ventilatory support devices, such as continuous positive airway pressure (CPAP) and non-invasive ventilation (NIV), has played a decisive role in the treatment of ARF secondary to CPE. The use of either CPAP2•7 or NIV8•11 has resulted in greater clinical improvements compared with standard medical therapy. Hypercapnia without chronic lung disease has been associated with poor outcomes in patients with CPE,12,13 particularly when PaCO2 is higher than 60mmHg.13 Although there is a strong indication for NIV in hypercapnic patients,11,12 the superiority of NIV over CPAP remains unclear, and hence, both have been recommended.14•31
NIV and CPAP have both been successfully used in CPE patients admitted to an intensive care unit (ICU).8•10,28 However, few trials have been published in the ICU setting.10,28 In addition, acute coronary syndrome (ACS) has been considered to be an exclusion criterion in several trials.10,11,18,19,28•31
The aim of the present study was to demonstrate that NIV performs better than CPAP in the management of CPE in an ICU setting. The primary outcome was a reduction in the need for endotracheal intubation in the NIV group. The secondary outcomes were duration of ventilation, ICU and hospital stay, ICU and hospital mortality, and 28-day mortality. The clinical and gasometric improvements, together with the rate of complications (renal failure, nosocomial infections), were all recorded. We also assessed the role of hypercapnia (PaCO2>45mmHg) on primary and secondary outcomes in patients with no underlying chronic lung disease.
MethodsA prospective, randomized study was conducted in a medical-surgical ICU from July 2007 to December 2010. The study protocols were approved by the local clinical research ethics committee. Written consent was required from all patients, or from their next of kin, before inclusion in the study. CPE patients aged 18 years or older admitted to the ICU from the emergency department (ED), a hospital ward, or the cardiac catheterization laboratory were included in the study. Cardiogenic pulmonary edema is defined as the presence of dyspnea, respiratory rate >25breaths/minute, the use of accessory respiratory muscles, cyanosis, cold sweats, bilateral crackles and/or wheezing on pulmonary auscultation, hypoxemia, hypertension, and a predominance of bilateral pulmonary infiltrates on chest radiography (if available).1 The potential causes of CPE are understood to be ACS with or without persistent ST-elevation, hypertensive emergency, valvulopathy, acute arrhythmia, endocarditis, or decompensation due to chronic heart failure.1 The exclusion criteria were: refusal to give informed consent, inability to cooperate, severe encephalopathy (Glasgow coma score <10), anatomical difficulty when adjusting the face mask, non-cardiogenic ARF (pneumonia, blunt chest trauma, or chronic obstructive pulmonary disease), respiratory or cardiac arrest on admission, together with the need for immediate intubation.15 Specific cardiac contraindications were also considered, including: cardiogenic shock on admission established by systolic blood pressure (SBP) <90mmHg, or dependence on vasoactive drugs (norepinephrine >0.5α/4g/kg/min). Hypercapnia was defined as partial pressure of carbon dioxide (PaCO2)>45mmHg.11,13 Patients with chronic obstructive pulmonary disease (COPD) or obstructive sleep apnea syndrome (OSA) were excluded for the analysis in the hypercapnic group.
MethodologyPatients were continuously monitored via electrocardiography and invasive or non-invasive blood pressure measurements. Blood oxygen was monitored using pulse oximetry, which estimates transcutaneous arterial oxygen saturation (SaO2), together with arterial blood samples for ABG analyses using the ABL 800 Flex (Radiometer⢢, Denmark, Copenhagen) blood gas analyzer which measures partial pressure of oxygen (PaO2), PaCO2, partial pressure of oxygen to fraction of oxygen ratio (PaO2/FiO2), and pH. Demographic data, comorbidities, and predicted mortality using the Simplified Acute Physiology Score 3 (SAPS 3), were all collected on admission. All vital signs and arterial blood gases (if available) were recorded at baseline and at 1, 2 and 8h after randomization. All complications arising during ICU stay were recorded, and patients were followed up for 28 days or until hospital discharge.
At the time of onset of CPE, either in the ED or on the ward, all patients received standard medical therapy (oxygen through a Venturi mask, morphine, intravenous nitroglycerin if SBP >160mmHg, together with loop diuretics) at the discretion of the attending physician. In the absence of clinical improvement (dyspnea, respiratory rate >25bpm, SaO2<90%), the patient was admitted to the ICU and assigned to the NIV group or the CPAP group, regardless of the treatment received in the ED. Patients already in the ICU at onset of CPE were randomized without preliminary medical treatment. Patients were assigned to each group using computer-randomized treatment allocations contained in a sealed envelope.
ProtocolThe NIV or CPAP procedure was explained to the recumbent patient. The oronasal mask was selected according to the patient's anatomy and subsequently adjusted using straps.15 Two alternative procedures were used. In the first, the CPAP was applied using a flow generator (WhisperFlow⢢, Caradyne, Ireland) capable of delivering 140L/min, with adjustable fractional inspired oxygen (FiO2) that ranged from 0.3 to 1.0. This was connected to the positive end-expiratory pressure (PEEP) valve attached to the face mask. In the second procedure, the Boussignac CPAP Flow Jet (Vygon¨r), Ecouen, France) system was used. The Boussignac valve takes gas from a single source and splits it in order to create 4 high flow jets. These jets converge in the chamber creating a turbulence which creates a virtual valve.30 A minimal PEEP initial level 5cmH2O was recommended for the first hour of CPAP, with subsequent increments (up to 15cmH2O) until clinical improvement was achieved. For NIV, a BiPAP Vision (Respironics Inc.¨r), Pennsylvania, USA) bilevel positive airway pressure system was used, setting the inspiratory positive airway pressure (IPAP) at the level (from 10 to 15cmH2O) required to achieve a tidal volume of approximately 8•10ml/kg. Expiratory positive airway pressure (EPAP) was set at a minimum of 5cmH2O for the first hour, gradually increasing (from 5 to 10cmH2O) until clinical improvement was observed.15 For both devices, FiO2 was applied to maintain SaO2 of 92%•94%. Both ventilatory devices were continuously applied until a clinical and/or a gasometric improvement was observed, at which time they were replaced by a Venturi mask with FiO2 of 0.4. Patients presenting ACS with persistent ST-elevation who met the criteria for reperfusion therapy underwent either thrombolysis or percutaneous coronary intervention, depending upon availability.
Ventilatory support was considered to be successful when patients experienced an improvement in ventilation (respiratory rate, improved patient•ventilator synchrony, respiratory muscle effort, SaO2 or ABG analysis) and in hemodynamic parameters (heart rate, blood pressure), thus ruling out intubation. The procedure was considered to have failed when it was necessary to remove the non-invasive support and proceed with intubation, based on the following criteria: no improvement in gas exchange or dyspnea, intolerance of CPAP or NIV, onset of ventricular arrhythmias, deteriorating level of consciousness (GCS<10), respiratory or cardiac arrest, or evolution toward cardiogenic shock. The decision to interrupt NIV, change FiO2 and IPAP/EPAP, and to intubate was taken by the attending physician. The decision to limit therapeutic effort in patients refractory to respiratory and hemodynamic measures, evolving possibly toward multiple organ failure, is taken by the attending physician.
Statistical AnalysesBased on previous studies,10,15 we hypothesized that the need for intubation could be reduced by 15% (19% in the CPAP [control] group vs. 4% in the NIV [study] group). The estimated sample size was 55 patients in each group (confidence interval [1∧α]=90% and power [1∧β]=85%). Data were compared using the Student's t-test or the Mann•Whitney test for quantitative variables and for parametric and non-parametric variables, respectively. For qualitative variables, we used the chi-square statistic or Fisher's exact test. Statistical significance was set at P<.05. Repeated measures ANOVA (with Bonferroni's correction) were performed to determine the influence of both ventilatory devices on ABG variables. The cumulative probability of survival was compared using the Kaplan•Meier estimation of survival and log-rank test to compare both of the groups. Intention-to-treat analysis was also performed. The data were analyzed using the statistical package SPSS 18.0.
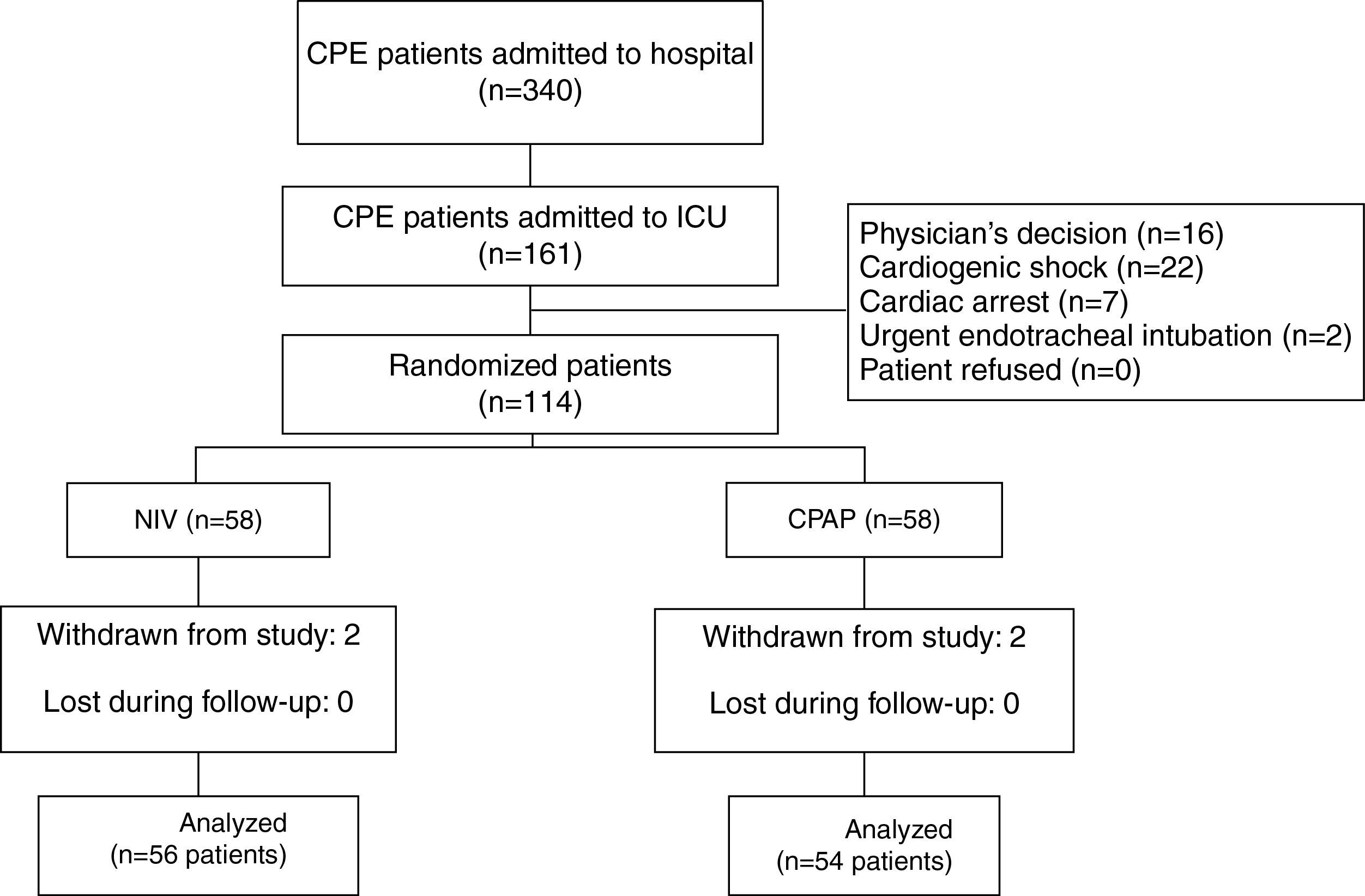
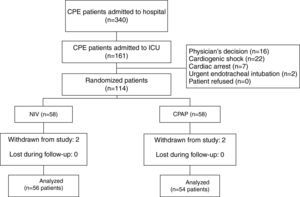
ResultsOf 161 potentially eligible patients (Fig. 1), 47 met the exclusion criteria. Thus, 56 out of 114 enrolled patients were assigned to NIV and 54 to CPAP. Four cases met exclusion criterion after allocation (2 in the NIV group and 2 in the CPAP group) due to community-acquired pneumonia, nosocomial pneumonia, abdominal sepsis and hypertensive emergency complicated with CPE, and hemorrhagic stroke, respectively. All were excluded from the analysis. Only 1 patient in the CPAP group underwent NIV treatment. Finally, 110 patients were included in the analysis.
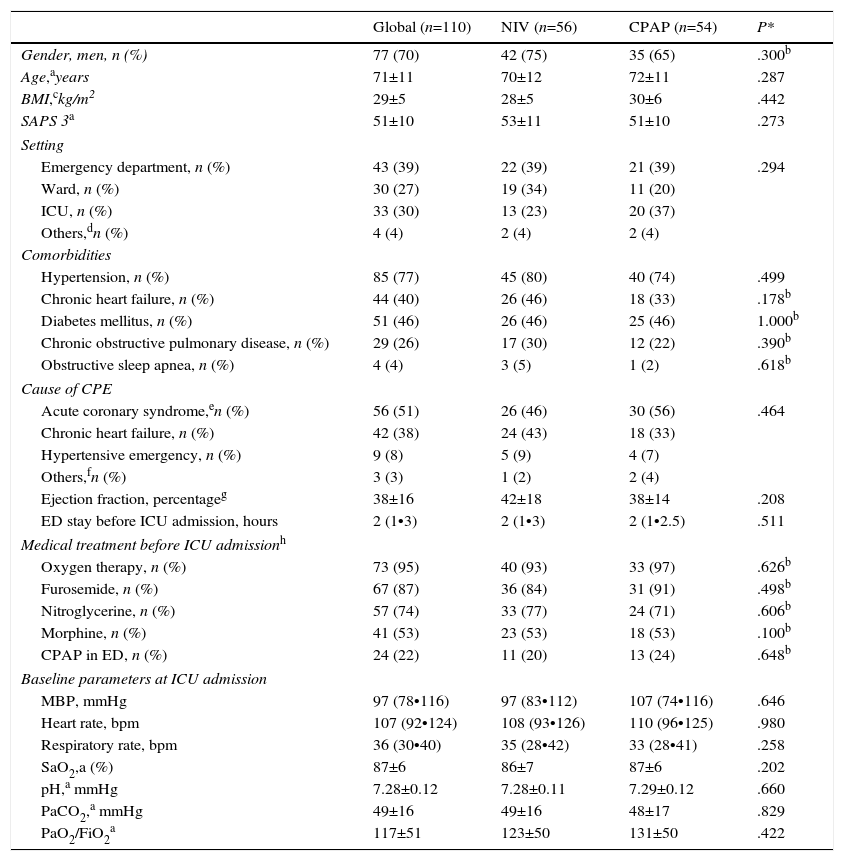
Onset of CPE (Table 1) occurred in the ED in the most cases. No differences were found between patients who developed CPE in the ED and in other settings in terms of age, sex, SAPS 3, comorbidities and hemodynamic and respiratory parameters. The main reason for CPE was complicated ACS and chronic heart failure (Table 1). Twenty-four patients received CPAP treatment during their stay in the ED. The comparison of physiological, clinical and gasometric variables between patients treated (n=24) and not treated (n=86) with CPAP prior to randomization did not show significant differences, except for the percentage of patients with diabetes mellitus (16 patients in the NIV group vs. 35 patients in the CPAP group, P=.036) (Table 1).
Baseline Demographic Characteristics, Comorbidities, and Medical Treatment Received Before Allocation to Treatment.
| Global (n=110) | NIV (n=56) | CPAP (n=54) | P* | |
|---|---|---|---|---|
| Gender, men, n (%) | 77 (70) | 42 (75) | 35 (65) | .300b |
| Age,ayears | 71±11 | 70±12 | 72±11 | .287 |
| BMI,ckg/m2 | 29±5 | 28±5 | 30±6 | .442 |
| SAPS 3a | 51±10 | 53±11 | 51±10 | .273 |
| Setting | ||||
| Emergency department, n (%) | 43 (39) | 22 (39) | 21 (39) | .294 |
| Ward, n (%) | 30 (27) | 19 (34) | 11 (20) | |
| ICU, n (%) | 33 (30) | 13 (23) | 20 (37) | |
| Others,dn (%) | 4 (4) | 2 (4) | 2 (4) | |
| Comorbidities | ||||
| Hypertension, n (%) | 85 (77) | 45 (80) | 40 (74) | .499 |
| Chronic heart failure, n (%) | 44 (40) | 26 (46) | 18 (33) | .178b |
| Diabetes mellitus, n (%) | 51 (46) | 26 (46) | 25 (46) | 1.000b |
| Chronic obstructive pulmonary disease, n (%) | 29 (26) | 17 (30) | 12 (22) | .390b |
| Obstructive sleep apnea, n (%) | 4 (4) | 3 (5) | 1 (2) | .618b |
| Cause of CPE | ||||
| Acute coronary syndrome,en (%) | 56 (51) | 26 (46) | 30 (56) | .464 |
| Chronic heart failure, n (%) | 42 (38) | 24 (43) | 18 (33) | |
| Hypertensive emergency, n (%) | 9 (8) | 5 (9) | 4 (7) | |
| Others,fn (%) | 3 (3) | 1 (2) | 2 (4) | |
| Ejection fraction, percentageg | 38±16 | 42±18 | 38±14 | .208 |
| ED stay before ICU admission, hours | 2 (1•3) | 2 (1•3) | 2 (1•2.5) | .511 |
| Medical treatment before ICU admissionh | ||||
| Oxygen therapy, n (%) | 73 (95) | 40 (93) | 33 (97) | .626b |
| Furosemide, n (%) | 67 (87) | 36 (84) | 31 (91) | .498b |
| Nitroglycerine, n (%) | 57 (74) | 33 (77) | 24 (71) | .606b |
| Morphine, n (%) | 41 (53) | 23 (53) | 18 (53) | .100b |
| CPAP in ED, n (%) | 24 (22) | 11 (20) | 13 (24) | .648b |
| Baseline parameters at ICU admission | ||||
| MBP, mmHg | 97 (78•116) | 97 (83•112) | 107 (74•116) | .646 |
| Heart rate, bpm | 107 (92•124) | 108 (93•126) | 110 (96•125) | .980 |
| Respiratory rate, bpm | 36 (30•40) | 35 (28•42) | 33 (28•41) | .258 |
| SaO2,a (%) | 87±6 | 86±7 | 87±6 | .202 |
| pH,a mmHg | 7.28±0.12 | 7.28±0.11 | 7.29±0.12 | .660 |
| PaCO2,a mmHg | 49±16 | 49±16 | 48±17 | .829 |
| PaO2/FiO2a | 117±51 | 123±50 | 131±50 | .422 |
CPAP: continuous positive airway pressure; NIV: noninvasive ventilation; CPE: cardiogenic pulmonary edema; SAPS: simplified acute physiology score; ICU: intensive care unit; ED: emergency department; MBP: mean blood pressure; SaO2: transcutaneous arterial oxygen saturation; PaO2/FiO2: partial pressure of oxygen to fraction of oxygen ratio.
NIV group: 1 patient from another hospital and 1 from cardiac catheterization laboratory; CPAP group: 1 patient from cardiac catheterization laboratory.
Patients with acute coronary syndrome with persistent ST-elevation indicated for reperfusion therapy (NIV in 8 patients vs. CPAP in 14 patients), acute coronary syndrome with myocardial infarction (NIV in 6 patients vs. CPAP in 6 patients), acute coronary syndrome without ST-elevation (NIV in 12 patients vs. CPAP in 10 patients).
One patient (2%) with CPE due to valvular decompensation in NIV group; 2 patients (4%) with CPE secondary to acute arrhythmia in CPAP group.
Taking to account treatment in ED and ward: n=38 patients in NIV group and n=34 patients in CPAP group.
At baseline, there were no significant differences between the NIV and CPAP groups (Table 1) in terms of age, gender, SAPS 3, cause of CPE and comorbidities. Blood oxygen levels did not differ between the groups (Table 1). There was no significant difference in length of stay in the ED before ICU admission. The median time from diagnosis of CPE to randomization was similar in both groups (30 [20•90] minutes in NIV vs. 30 [17•75] minutes in CPAP, P=.571).
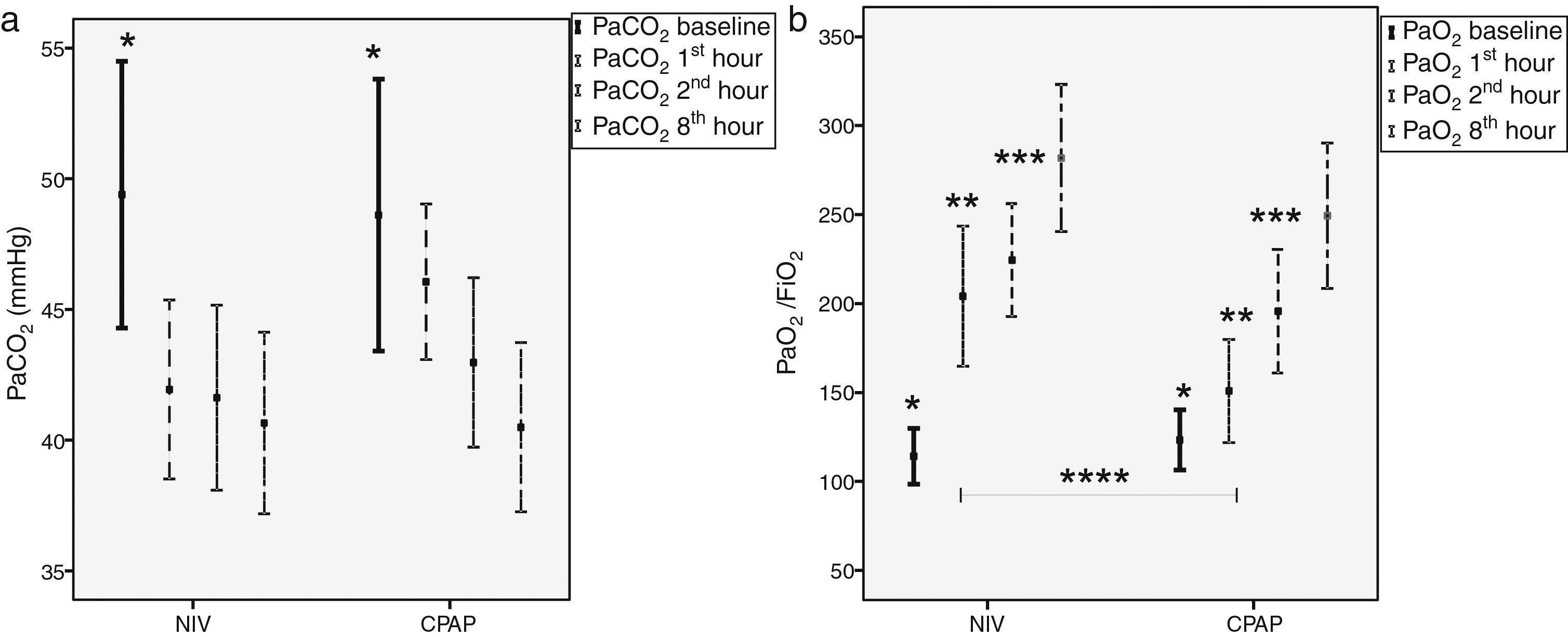
Mean IPAP levels for the first 60min were 14±4cmH2O and 6±1cmH2O for EPAP in the NIV group, respectively. In the CPAP group, mean pressure was 7±2cmH2O. Both treatments led to significant reductions in PaCO2 and, simultaneously, significant increments in PaO2/FiO2 ratio (Fig. 2). During the first hour of therapy, PaO2/FiO2 ratio improved to a greater extent in the NIV group vs, the CPAP group (205±112 vs. 150±84 in CPAP, P=.02), but these differences were equaled over time (Fig. 2b).
Time-course of carbon dioxide partial pressure (PaCO2) and oxygen partial pressure to fraction of oxygen ratio (PaO2/FiO2) comparing NIV to CPAP (a) Left figure shows PaCO2 comparing NIV (n=26) to CPAP (n=25). (b) Right figure shows PaO2/FiO2 comparing NIV (n=21) to CPAP (n=20); Mean±SD Bonferroni's correction *P≤.05 baseline with respect to 60, 120, 480min; **P≤.05 at 60min with respect to 120•480min; ***P≤0.003•120min with respect to 480min; ****NIV vs. CPAP 60min of ventilation (P=.02). NIV: non-invasive ventilation; CPAP: continuous positive airway pressure; n: number of patients.
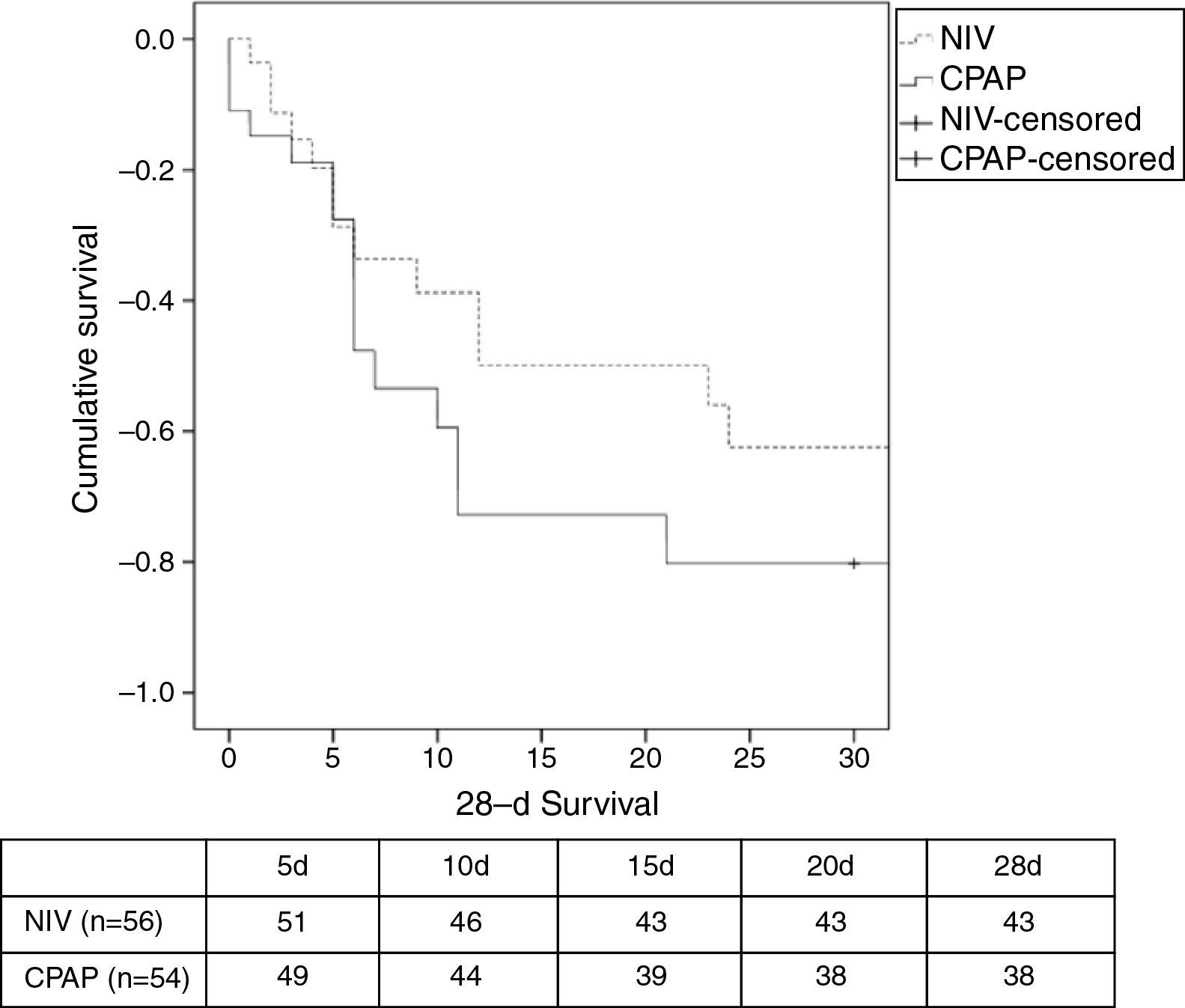
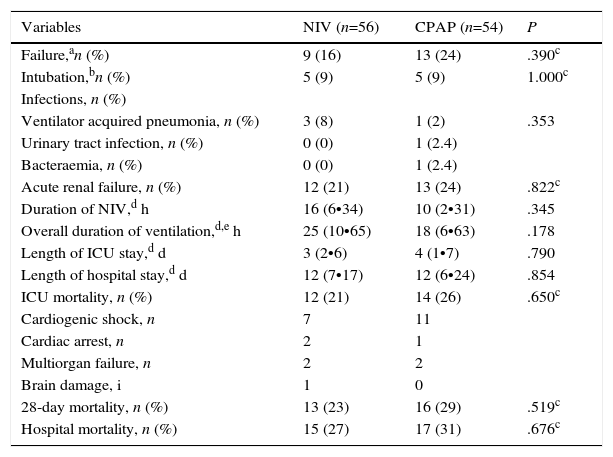
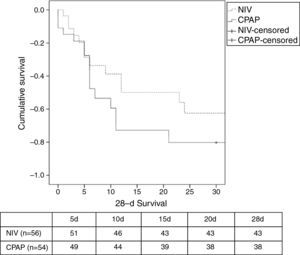
There was a similar (but not significantly) number of endotracheal intubations and fewer complications in the NIV group vs. the CPAP group (Table 2). There were no differences in the duration of ventilation, length of ICU stay, hospital stay, or mortality (Table 2, Fig. 3). No patients were readmitted for CPE during their hospital stay.
Analysis of Primary and Secondary Outcomes of the Study.
| Variables | NIV (n=56) | CPAP (n=54) | P |
|---|---|---|---|
| Failure,an (%) | 9 (16) | 13 (24) | .390c |
| Intubation,bn (%) | 5 (9) | 5 (9) | 1.000c |
| Infections, n (%) | |||
| Ventilator acquired pneumonia, n (%) | 3 (8) | 1 (2) | .353 |
| Urinary tract infection, n (%) | 0 (0) | 1 (2.4) | |
| Bacteraemia, n (%) | 0 (0) | 1 (2.4) | |
| Acute renal failure, n (%) | 12 (21) | 13 (24) | .822c |
| Duration of NIV,d h | 16 (6•34) | 10 (2•31) | .345 |
| Overall duration of ventilation,d,e h | 25 (10•65) | 18 (6•63) | .178 |
| Length of ICU stay,d d | 3 (2•6) | 4 (1•7) | .790 |
| Length of hospital stay,d d | 12 (7•17) | 12 (6•24) | .854 |
| ICU mortality, n (%) | 12 (21) | 14 (26) | .650c |
| Cardiogenic shock, n | 7 | 11 | |
| Cardiac arrest, n | 2 | 1 | |
| Multiorgan failure, n | 2 | 2 | |
| Brain damage, i | 1 | 0 | |
| 28-day mortality, n (%) | 13 (23) | 16 (29) | .519c |
| Hospital mortality, n (%) | 15 (27) | 17 (31) | .676c |
CPAP: continuous positive airway pressure; CPE: ICU: intensive care unit; cardiogenic pulmonary edema; LTE: limitation of therapeutic effort; NIV: noninvasive ventilation.
Causes of failure: no reduction of dyspnea (2 patients in NIV group vs. 2 patients in CPAP group), cardiac or respiratory arrest (2 patients in NIV group vs. 2 patients in CPAP group), lack of improvement in gas exchange (2 patients in NIV group vs. 2 patients in CPAP group), deteriorating level of consciousness (1 patient in NIV group), intolerance of NIV (1 patient in NIV group), cardiogenic shock (1 patient in NIV group vs. 7 patients in CPAP group).
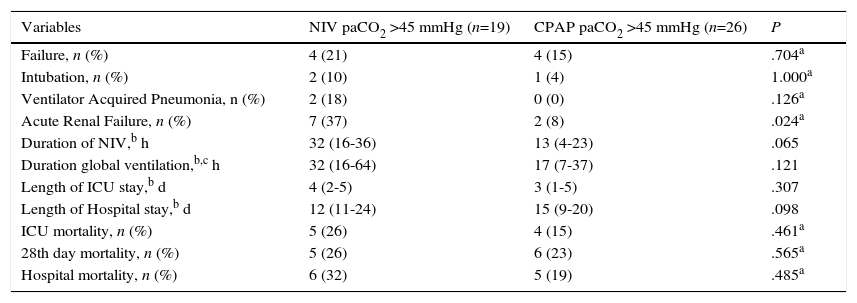
In the hypercapnic group of patients without chronic lung disease, no significant differences between the NIV and CPAP groups were found, except for a higher rate of acute renal failure in the NIV group (37% in NIV group vs. 8% in CPAP group, P=0.024) (Table 3).
Analysis of primary and secondary outcomes in the hypercapnic group.
| Variables | NIV paCO2 >45 mmHg (n=19) | CPAP paCO2 >45 mmHg (n=26) | P |
|---|---|---|---|
| Failure, n (%) | 4 (21) | 4 (15) | .704a |
| Intubation, n (%) | 2 (10) | 1 (4) | 1.000a |
| Ventilator Acquired Pneumonia, n (%) | 2 (18) | 0 (0) | .126a |
| Acute Renal Failure, n (%) | 7 (37) | 2 (8) | .024a |
| Duration of NIV,b h | 32 (16-36) | 13 (4-23) | .065 |
| Duration global ventilation,b,c h | 32 (16-64) | 17 (7-37) | .121 |
| Length of ICU stay,b d | 4 (2-5) | 3 (1-5) | .307 |
| Length of Hospital stay,b d | 12 (11-24) | 15 (9-20) | .098 |
| ICU mortality, n (%) | 5 (26) | 4 (15) | .461a |
| 28th day mortality, n (%) | 5 (26) | 6 (23) | .565a |
| Hospital mortality, n (%) | 6 (32) | 5 (19) | .485a |
CPE: cardiogenic pulmonary edema; NIV: noninvasive ventilation; CPAP: continuous positive.
This study has shown that ICU patients admitted as a result of CPE and treated with either NIV or CPAP required a similar rate of intubation. Both clinical parameters and gasometric values improved in both groups, accompanied by an identical number of complications. Improvement in PaO2/FiO2 ratios in the first hours was faster with NIV than with CPAP, although these differences subsequently equalized. In hypercapnic patients without pulmonary pathology, there is no clinical benefit observed in the use of NIV over CPAP.
Ours is one of the few studies carried out in the ICU setting, and accurately reflects real clinical practice, where either NIV or CPAP was applied in patients with ACS, in accordance with current recommendations. Baseline characteristics were similar in both groups, regardless of the origin of the patient, the medical treatment administered prior to ICU admission, and whether or not ventilatory support had already been initiated. The absence of significant differences between the groups prior to randomization ensures the homogeneity of the sample.
The increased intrathoracic pressure generated by CPAP had both respiratory and hemodynamic effects. CPAP increased functional residual capacity and lung compliance. This was accompanied by smaller transpulmonary pressure swings during the respiratory cycle, and therefore less work of breathing. At the same time, such positive pressures reduced venous return and left ventricular afterload, thereby reducing left ventricular transmural pressure and increasing cardiac output.32,33 NIV had similar effects, with IPAP unloading respiratory muscles during ventilation.33
A theoretical benefit of NIV compared to medical treatment was observed in patients with persistent hypercapnia together with severe decompensation of a previously chronic obstructive pulmonary disease due to the CPE.11 Accordingly, a randomized trial in patients with CPE showed that NIV improved carbon dioxide clearance more effectively than CPAP12. However, these results are not consistent with other studies18,29,30 in which NIV and CPAP led to similar improvements in hemodynamics, respiratory mechanics and gas exchange. Contrary to the aforementioned study,12 a retrospective observational study demonstrated that CPAP to rapidly increase pH can be successfully applied in the presence of metabolic or respiratory acidosis.34 Moreover, failure and mortality rates were similar in acidotic and non-acidotic patients.34 This indicates that CPAP improves ventilatory patterns sufficiently to reduce the level of PaCO2 despite respiratory acidosis. Our results are supported by several studies reporting faster improvement in PaO2/FiO2 ratios with NIV vs. CPAP,12,26,31 but differ from studies finding no such differences.18,19,30,31
In our study, the NIV failure rate was higher than in studies conducted in the ED,18,19,29•31 but similar to other ICU studies.26•28 However, the percentage of intubation was lower because of limitation of therapeutic effort. Surprisingly, the percentage of cardiogenic shock was higher in the CPAP group, but this did not influence the results. Similarly, a study comparing CPAP with proportional assisted ventilation (PAV) for CPE in the ICU showed a higher intubation rate in PAV, although the differences were not significant (29% vs. 21% in the CPAP group, P=0.71).28 Two prospective French26 and Spanish27 studies on NIV found a similar need for endotracheal intubation (16% and 15%, respectively). Thus, the intubation rate in our study appears to differ depending on whether the patient was admitted from the ED or presented CPE while in the ICU. These differences cannot be attributed to differences in pressure levels, since these were similar to those used in other studies.18,19,29•31 One possible explanation could be a selection bias in respect of patients admitted from the ED, since only patients with incomplete CPE resolution were admitted to the ICU. Another hypothesis is that it may have been influenced by the inclusion of ACS patients with persistently elevated ST at increased risk of cardiogenic shock. Contrary to established recommendations,23 the use of CPAP or NIV in ACS patients did not increase the failure rate, as half of our population presented ACS, but 7% to 14% required intubation. A retrospective study on the use of either CPAP or NIV in CPE secondary to ACS and non-ACS did not find significant differences in the overall intubation rate.35 These findings suggest that the recommendation to avoid non-invasive devices in patients scheduled for revascularization techniques should be reconsidered.
Mortality in our study was clearly higher than in some previous studies,18,19,29•31 but was consistent with SAPS 3 predicted mortality and consistent with ICU mortality as reported by Rusterholtz et al.,28 with 23% in the PAV group vs. 21% in the CPAP group, respectively (P=.99). According to our data, ICU mortality in one of the afore-mentioned surveys was high (30%).27 In terms of other complications, we observed a higher rate of renal failure in the CPAP group. This could have prolonged ICU stay, but did not cause in a significant difference in mortality when compared to the NIV group.
Hypercapnia is frequent in patients with acute pulmonary edema, and has been associated with worse clinical outcome in various studies.11,13,29,31 Our findings in this respect are consistent with other authors, insofar as neither NIV nor CPAP were superior in hypercapnic patients.29,31 These data justify systematically ruling out NIV in hypercapnic patients with no chronic pulmonary disease, despite elevated PaCO2 levels. Similarly to other studies, in our study duration of both NIV and CPAP ventilation was longer in the hypercapnic group.13,29,31
Unlike other clinical trials,36 our study protocol did not allow switching from CPAP to NIV; however, this was violated in the case of 1 patient who was switched from CPAP to NIV in order to avoid endotracheal intubation. The study of reference was a large randomized controlled study that compared oxygen vs. NIV plus CPAP, reporting a similar failure rate in both groups (2.8 vs. 2.9% P=.9).36 A subsequent review22 attempted to explain the low rate of intubation in that study,36 attributing it to the crossover of patients from oxygen to CPAP, or in particular, to NIV.
Our study has several limitations, as it was carried out in a single unit and some patients had been treated with CPAP in the ED. From a methodological point of view, several limitations were imposed in the design: first, patients with ACS were included, although ACS was an exclusion criterion in previous studies,10,11,18,19,29•32 and secondly, unlike recent studies, a third arm receiving either oxygen therapy or high-flow oxygen was not considered.36,37 This was because our study was designed prior to the study of reference, and was based on current recommendations.1,14•16 Finally, in the analysis of hypercapnic patients, COPD and OSA patients were excluded, and patients with obesity could not be excluded given the small number of patients in whom BMI could be recorded.
ConclusionIn summary, either NIV or CPAP can be used in the ICU; both are equally effective and achieve a similar clinical response. In hypercapnic patients with no pulmonary disease, there is no clinical benefit in the use of NIV over CPAP.
Conflict of InterestThe authors declare that they have no conflict of interest.
Initial results of the study were presented in XLIV Congreso Nacional de la Sociedad Española de Medicina Intensiva y Unidades Coronarias (SEMICYUC), Valladolid 2009, Spain. Med Intensiva 2009; 33 (espec cong): 81•111.