Viral lower respiratory tract infections frequently cause morbidity and mortality in children. The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic led to isolation and hygiene measures, resulting in decreased respiratory virus transmission and pediatric admissions. This study aimed to assess the impact of these measures and their uplifting on respiratory virus circulation in children before and during the SARS-CoV-2 pandemic (January 2017-December 2022).
MethodsWe conducted a weekly time series analysis of multiple virus molecular assays in children. This included those admitted to a university reference hospital's Pediatric Intensive Care Unit (PICU) and those with risk pathologies exhibiting fever and/or respiratory symptoms. We included patients aged 0-18 years residing in Catalonia and adjusted the positive results to account for diagnostic effort.
ResultsWe performed a total of 2991 respiratory virus tests during the period. Confinement significantly decreased the detection of all viruses, especially Rhinovirus (RV). After the deconfinement of children, the viral detection trend remained stable for all viruses, with no short-term impact on virus transmission. The mandatory implementation of facemasks in those aged ≥6 years led to decreased viral circulation, but we observed an influenza virus rebound after facemask removal. At that time, we also noticed an interrupted drop in the detection rates of RV and respiratory syncytial virus (RSV). The reopening of schools led to a progressive increase in viral detections, especially of Rhinovirus.
ConclusionNon-pharmacological interventions significantly impact the circulation of respiratory viruses among children. We observed these effects even when some measures did not specifically target preschool-aged children.
Lower respiratory tract infections (LRTI) are a leading cause of morbidity and mortality in children.1 In Spain, the global hospitalization rate for severe acute respiratory infection ranges from 10 to 30 cases per 100,000 inhabitants. For children under 5 years, the rate varies between 10 and 170 cases per 100,000 inhabitants annually.2 These infections are primarily viral, involving agents such as RSV, Rhinovirus/Enteroviruses and others. Severity could be influenced by environmental, epidemiological, microbiological, and host susceptibility factors.
The onset of the SARS-CoV-2 pandemic in March 2020, raised concerns about its overlap with seasonal respiratory infections, leading to potential care burdens.3 In response, health authorities worldwide implemented community strategies such as hand hygiene, face covering, and social distancing, which were associated with a reduction in respiratory virus transmission and pediatric admissions due to viral infections.4 This reduction remained consistent across countries, despite the different pandemic trajectories.5
This study aims to assess the impact of these measures and their relaxation on respiratory virus circulation in children before and during the SARS-CoV-2 pandemic (January 2017-December 2022).
MethodsData. Inclusion and exclusion criteriaDuring the SARS-CoV-2 pandemic in Spain, different autonomous communities implemented their own specific isolation measures. Weekly time series data of viral detections within the institutional respiratory virus surveillance program were analyzed. This program includes the molecular detection of multiple respiratory viruses in all children who require PICU admission, as well as in those admitted or treated as outpatients due to fever and/or respiratory symptoms (cough, nose throat, rhonchi or wheezing) with hematologic malignancy, primary or secondary immunosuppression, chronic cardiac disease with cyanosis or hemodynamically significant, and chronic respiratory conditions. To ensure consistency in isolation measures, only patients residing in Catalonia were considered, as the health authorities’ recommendations varied across different regions of Spain.
SettingOn 13th March 2020, the Government of Catalonia mandated the closure of schools in an effort to contain the spread of SARS-CoV-2. As a result, 1,565,478 students were required to stay at home during the first wave of the pandemic. Mandatory use of face masks in conjunction with other social distancing measures were introduced in the 20th week.
Schools reopened on 14th September 2020, marking the beginning of the 2020-2021 academic year. Strict non-pharmacological interventions (NPI) were implemented, including hand washing, mandatory use of face masks for children older than 5 years, social distancing measures, and organization of children and teachers into bubble groups. The goal was to maintain consistent groups of individuals to facilitate contact tracing. Additionally, enhanced ventilation was introduced in the classrooms. Other public health practices, such as screening and quarantining the entire group when a positive case was detected, were also adopted.
On 17th week of 2022, the mandatory use of masks and other measures, such as isolation by bubble groups or group quarantines, were withdrawn.6
Data on the strictness of lockdown policies restricting people's behaviour in Catalonia were sourced from the Health Department of Generalitat of Catalonia.7 The study spanned from January 2017 to December 2022, covering a wide period before and during the SARS-CoV-2 pandemic at the Sant Joan de Déu (SJD) Hospital in Barcelona. This university center, one of the largest children's and women's hospitals in Spain and Europe, has 314 beds and 161 outpatient-consulting rooms. It serves 350,000 inhabitants under the age of 18 as a territorial hospital, but it also acts as a pediatric reference hospital in Catalonia, with a total population of 7,653,845 inhabitants, of whom 1,384,382 were under 18. The hospital captures around 20% of pediatric admissions of this autonomous community.
Study periodsFive distinct periods were analyzed in terms of the epidemiological measures applied, depending on the circumstances:
- 1.
Pre-pandemic (weeks 1/17-11/20): This is the time before the SARS-CoV-2 pandemic emerged, when no specific pandemic-related measures were in place.
- 2.
Confinement (weeks 12/20-22/20): Strict confinement measures to control the spread of SARS-CoV-2, including staying indoors and the closure of non-essential businesses and educational institutions, were implemented.
- 2.1
Children deconfinement (weeks 17/20-24/20): There was a progressive relaxation of the confinement measures, allowing people to leave their homes and open shops and restaurants.
- 3.
Introduction of compulsory masks (weeks 23/20-38/20): Strict confinement was ended, and the authorities introduced mandatory mask-wearing, in addition to other social distancing measures to curb the transmission of the virus.
- 4.
Reopening of schools (weeks 39/20-16/22): As the pandemic situation improved, schools were allowed to reopen with strict NPI, including handwashing, mask use for children above 5 years, social distancing, and the organization of children and teachers into bubble groups.
- 5.
Ending of compulsory mask use (weeks 17/22-52/22): The mandatory use of masks was withdrawn, and other measures, such as isolations by bubble groups or quarantining the whole group if a positive case was detected, were relaxed.
The analysis of these distinct periods would provide valuable insights into the impact of different epidemiological measures on the circulation of respiratory viruses in the pediatric population before and during the SARS-CoV-2 pandemic.
LaboratoryMolecular assays (polymerase chain reaction [PCR]) were used for the detection of respiratory viruses. BioFire Filmarray® respiratory panel (bioMérieux, Salt Lake City, UT, USA) and QIAstat-Dx® respiratory panel (QIAGEN, Hilden, Germany) were utilized to detect multiple respiratory viruses, with weekly positive PCR results collected for Adenovirus (AdV), Non-SARS-CoV-2 human coronaviruses (hCoV-NL63, hCoV-HKU1, hCoV-229E and hCoV-OC43), human metapneumoviruses (HMPV), influenza A/B (VIA/B), parainfluenza viruses (PIV types 1-4), RV and RSV. Less frequent detection of Parainfluenza 1-4, Bocavirus, Bordetella pertussis, Bordetella parapertussis, Chlamydophila pneumoniae, Mycoplasma pneumoniae, Legionella pneumophila was recorded under the category of “others”. The number of positive results was adjusted for diagnostic effort, which was defined as the ratio of each positive viral PCR (cases) to the total number of PCR tests performed for each pathogen.
Statistical analysisComparisons were made between different periods based on the NPI measures implemented by health authorities. Continuous normal distributed variables were described as means with their 95% confidence intervals and compared using ANOVA. For non-normally distributed data, median values and interquartile ranges were provided and the Kruskal-Wallis test was used for comparisons. Correlations between weekly circulation of the main respiratory viruses, adjusted for diagnostic effort, were analyzed using the Pearson correlation test.
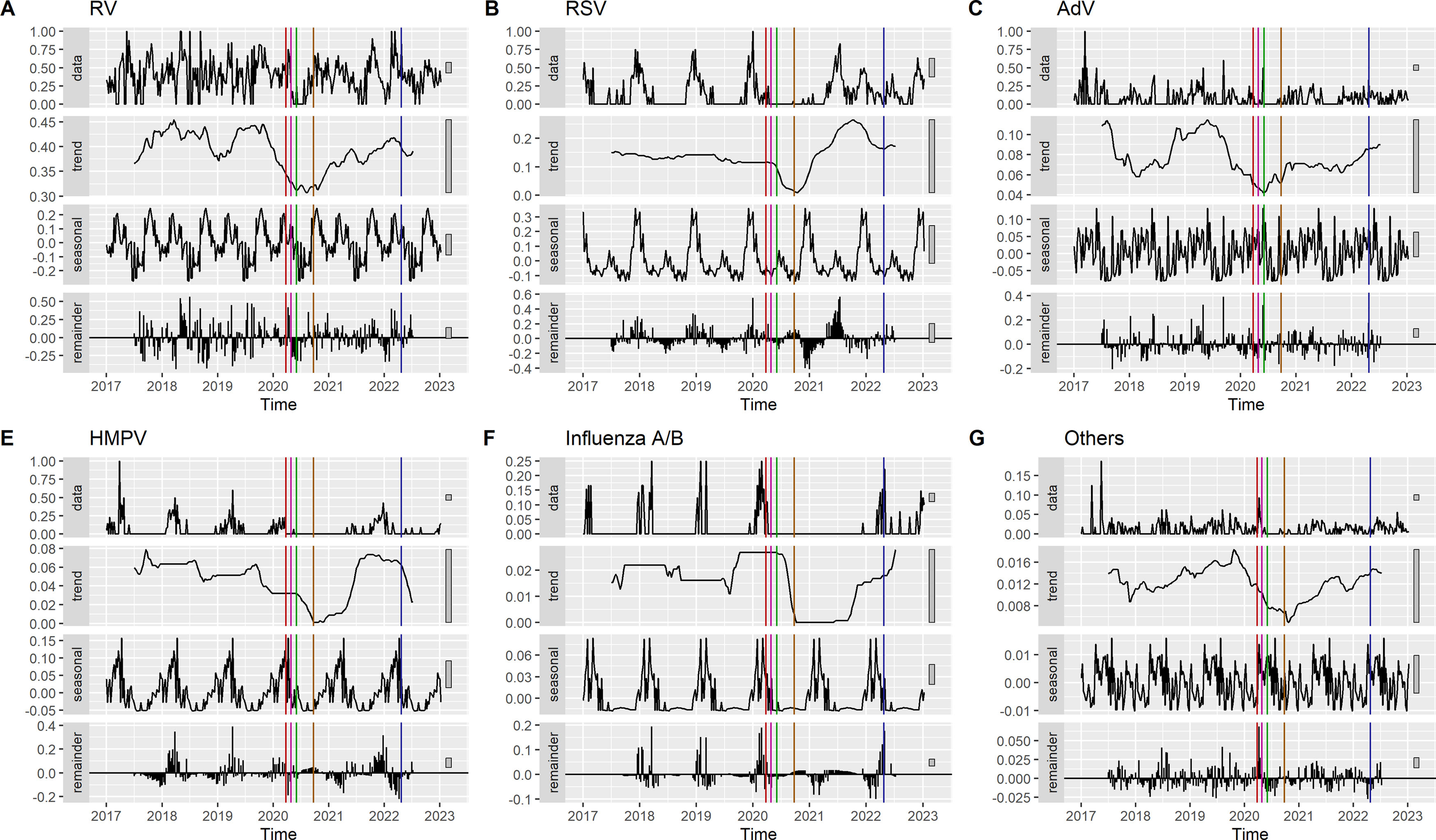
To visualize the impact of the implementation and removal of NPI, an analysis of deseasonalized time-series data using R software v 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria) and the tseries library (v0.10-54) was conducted. Four graphs were produced for each viral detection: adjusted detections accounting for diagnostic effort (positivity rate), trend to identify sustained changes over time, seasonal expected data, and residuals to detect unexplained fluctuations. Trend curves were used to identify changes in slope after each NPI was implemented or removed. In addition, we further analyzed short-term changes, specifically those within the two months following the measure's implementation, using a different methodology. This method provides a quantitative measurement and employs the CausalImpact library (v1.3.0).8 CausalImpact gathers time series data before each intervention, constructs a Bayesian structural time series model, and uses it to predict potential post-intervention trends. These predicted trends are then compared with the actual data to identify any discrepancies.9
Statistical analysis was performed using R software v 4.3.2 and the following packages: tseries (v0.10-54), corrplot (v0.92) and CausalImpact (v1.3.0). P-values less than 0.05 were considered significant.
Ethics approvalThis study was approved by the Ethics Committee and Institutional Review Board of the Sant Joan de Déu Hospital (PI179-21) and informed consents from patients’ parents were waived.
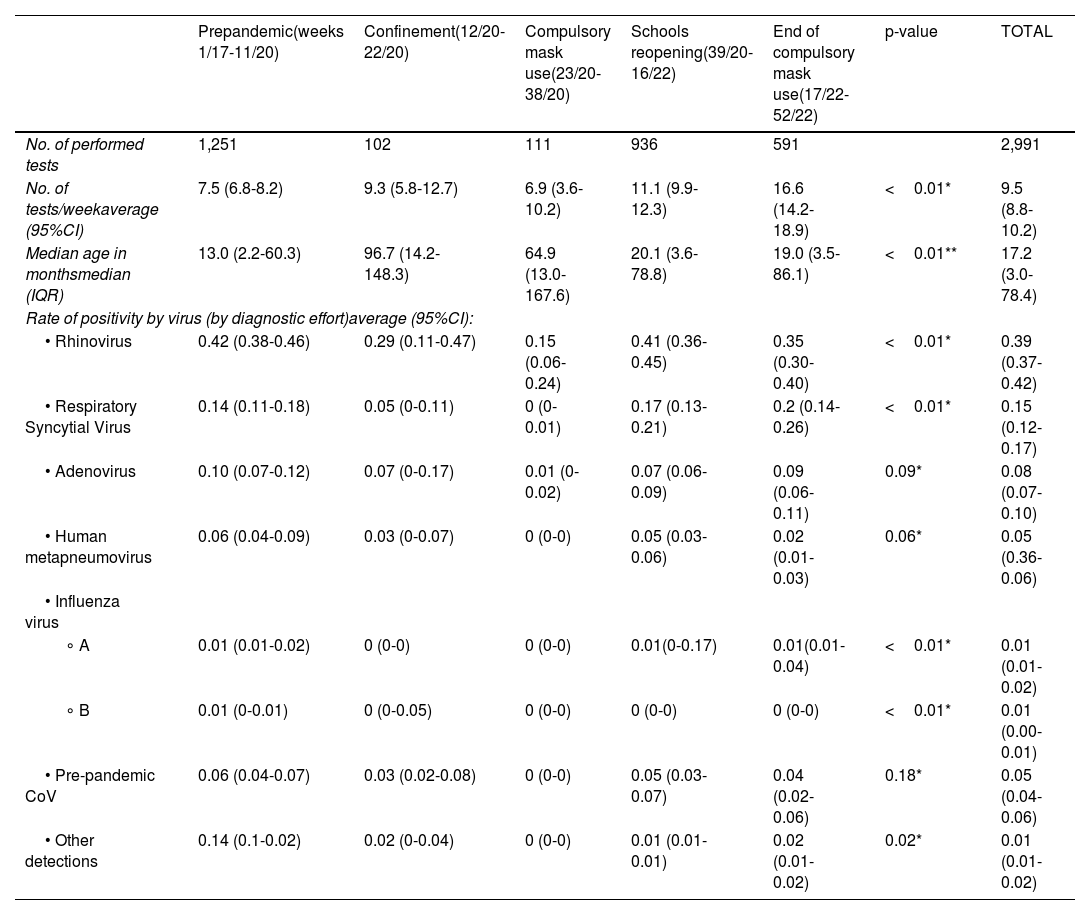
ResultsA total of 2,991 respiratory virus tests were performed in children during the study period. 1,251 tests were performed during the pre-pandemic phase, 213 during confinement and mandatory mask use, and 1,527 tests during the relaxation of NPI (reopening schools and ending mask mandates). The median age of the patients tested was 17.2 months (interquartile range [IQR]: 3.0-78.4), and statistically significant differences were found between the different periods, with older ages during confinement and the period of mandatory mask use. The average number of tests per week was 9.5 (8.8-10.2), being higher during the period of school reopening and the period of confinement, 11.1 (9.9-12.3) and 16.6 (14.2-18.9), respectively (Table 1). In relation to the rate of positivity by virus, globally RV was the most detected virus (0.39 [0.37-0.42]); followed by RSV and AdV, with rates of 0.15 (0.12-0.17) and 0.08 (0.07-0.10), respectively. Other less frequent viruses were HMPV, non-SARS-CoV-2 hCoV, Influenza A/B, and other detections. Positivity rates for each virus, considering the different phases, are detailed in Table 1.
General data overview and % positivity adjusted by diagnostic effort.
| Prepandemic(weeks 1/17-11/20) | Confinement(12/20-22/20) | Compulsory mask use(23/20-38/20) | Schools reopening(39/20-16/22) | End of compulsory mask use(17/22-52/22) | p-value | TOTAL | |
|---|---|---|---|---|---|---|---|
| No. of performed tests | 1,251 | 102 | 111 | 936 | 591 | 2,991 | |
| No. of tests/weekaverage (95%CI) | 7.5 (6.8-8.2) | 9.3 (5.8-12.7) | 6.9 (3.6-10.2) | 11.1 (9.9-12.3) | 16.6 (14.2-18.9) | <0.01* | 9.5 (8.8-10.2) |
| Median age in monthsmedian (IQR) | 13.0 (2.2-60.3) | 96.7 (14.2-148.3) | 64.9 (13.0-167.6) | 20.1 (3.6-78.8) | 19.0 (3.5-86.1) | <0.01** | 17.2 (3.0-78.4) |
| Rate of positivity by virus (by diagnostic effort)average (95%CI): | |||||||
| • Rhinovirus | 0.42 (0.38-0.46) | 0.29 (0.11-0.47) | 0.15 (0.06-0.24) | 0.41 (0.36-0.45) | 0.35 (0.30-0.40) | <0.01* | 0.39 (0.37-0.42) |
| • Respiratory Syncytial Virus | 0.14 (0.11-0.18) | 0.05 (0-0.11) | 0 (0-0.01) | 0.17 (0.13-0.21) | 0.2 (0.14-0.26) | <0.01* | 0.15 (0.12-0.17) |
| • Adenovirus | 0.10 (0.07-0.12) | 0.07 (0-0.17) | 0.01 (0-0.02) | 0.07 (0.06-0.09) | 0.09 (0.06-0.11) | 0.09* | 0.08 (0.07-0.10) |
| • Human metapneumovirus | 0.06 (0.04-0.09) | 0.03 (0-0.07) | 0 (0-0) | 0.05 (0.03-0.06) | 0.02 (0.01-0.03) | 0.06* | 0.05 (0.36-0.06) |
| • Influenza virus | |||||||
| ∘ A | 0.01 (0.01-0.02) | 0 (0-0) | 0 (0-0) | 0.01(0-0.17) | 0.01(0.01-0.04) | <0.01* | 0.01 (0.01-0.02) |
| ∘ B | 0.01 (0-0.01) | 0 (0-0.05) | 0 (0-0) | 0 (0-0) | 0 (0-0) | <0.01* | 0.01 (0.00-0.01) |
| • Pre-pandemic CoV | 0.06 (0.04-0.07) | 0.03 (0.02-0.08) | 0 (0-0) | 0.05 (0.03-0.07) | 0.04 (0.02-0.06) | 0.18* | 0.05 (0.04-0.06) |
| • Other detections | 0.14 (0.1-0.02) | 0.02 (0-0.04) | 0 (0-0) | 0.01 (0.01-0.01) | 0.02 (0.01-0.02) | 0.02* | 0.01 (0.01-0.02) |
CI, confidence interval; IQR, interquartile range
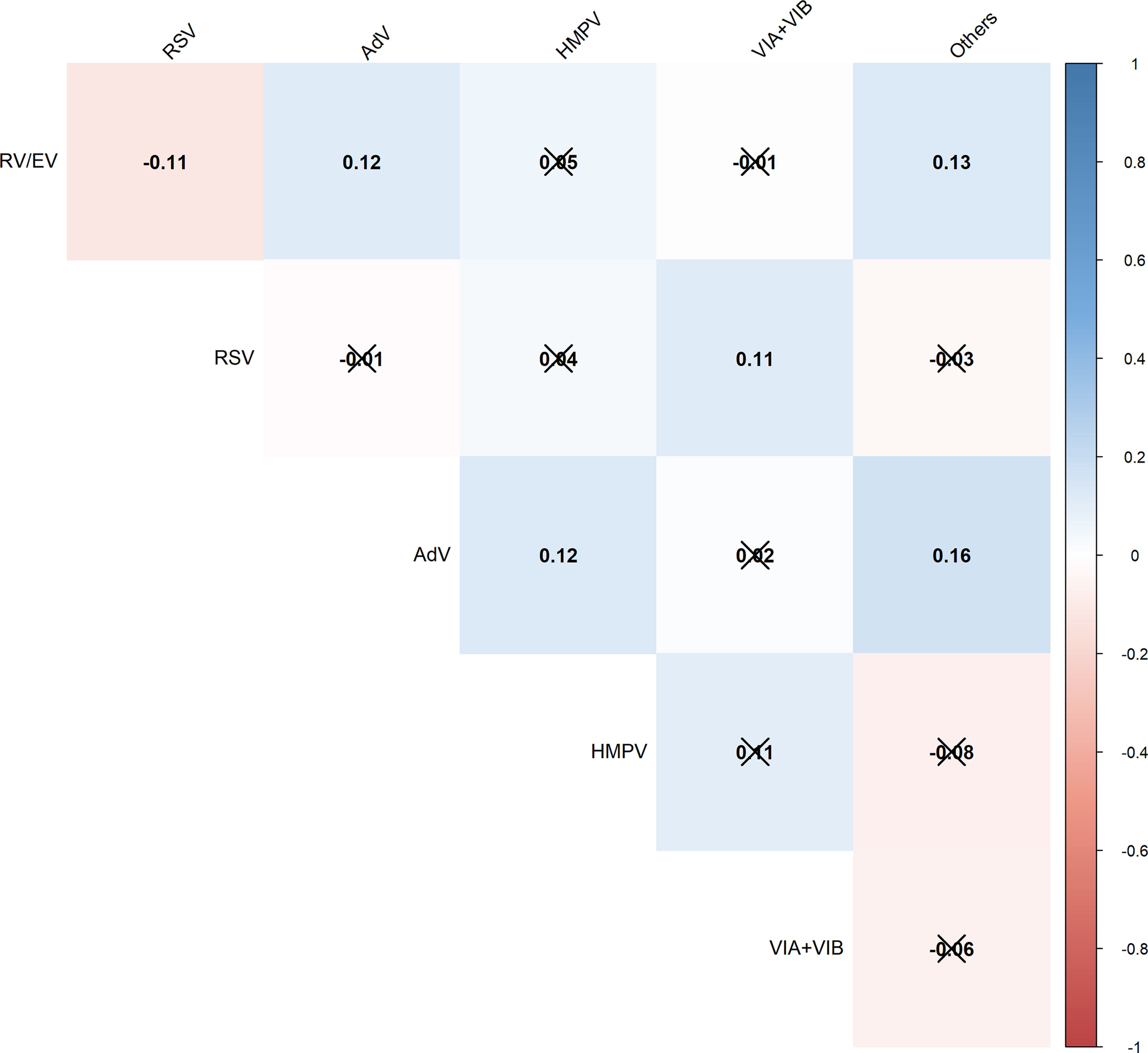
The correlation between the simultaneous weekly detection of different viruses is represented in Fig. 1.
Heatmap showing correlations (Pearson correlation coefficient) between weekly simultaneous detections (co-circulation).
Not significant correlations (p≥0.05) are struck through.
RV/EV, Rhinovirus/Enteroviruses; RSV, Respiratory Syncytial Virus; AdV, Adenovirus; HMPV, human Metapneumoviruses; VIA/B, Influenza A/B viruses.
Co-circulation was observed between RV, AdV, and other detections; RSV and Influenza viruses; and AdV, HMPV and other detections. On the other hand, a negative correlation in co-circulation was observed between RV and RSV.
Viral detections in relation to non-pharmacological intervention measuresThere was a significant decrease in viral detections following population confinement, which was the most restrictive NPI (Fig. 2, red line). However, this decrease was not immediately observed except for RV, AdV, and other less common viruses. Since this measure coincided with the end of RSV, HMPV, and Influenza seasons, the true impact of this measure on the transmission of these three viruses might be underestimated. The trend for all viral detections remained stable even after children's deconfinement (pink line). The implementation of compulsory masks (green line) immediately changed the trend of detections of RSV, HMPV and Influenza A/B. Despite maintaining this measure, the initiation of school activities (brown line) was followed by a slow and steady increase in RV, AdV, and less common viruses. Seasonal peaks of RSV, HMPV, and Influenza occurred later than expected. Influenza detections significantly increased after the compulsory mask period ended (blue line). Additionally, a drop in the detection rates of RV and RSV was interrupted at that time, and detections slightly increased.
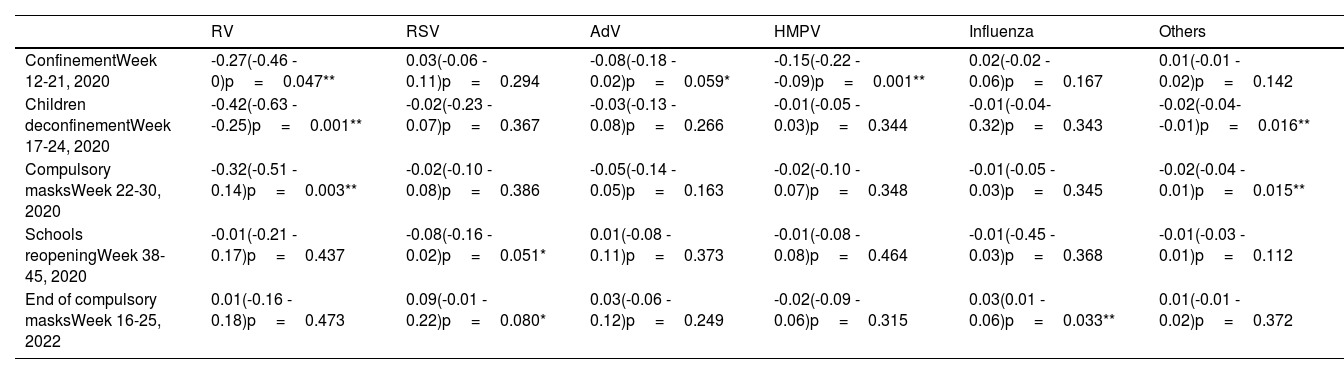
The results of the interrupted time-series analysis are presented in Table 2. The results showed a significant decrease in RV and HMPV detections following confinement, as well as a tendency to a significant reduction in AdV. During the period of children's deconfinement and the introduction of the compulsory mask, a lower detection rate of all viruses was observed, which was statistically significant for RV and other detections. The start of the 2020-21 school year did not lead to an immediate increase in viral detections. Lastly, the end of mandatory masks was linked to an increase in influenza detections, while RSV detection slightly increased after this measure.
Average absolute effect on the weekly virus detection rates, as determined by an interrupted time-series analysis conducted up to 2 months after the implementation of NPI measures.
| RV | RSV | AdV | HMPV | Influenza | Others | |
|---|---|---|---|---|---|---|
| ConfinementWeek 12-21, 2020 | -0.27(-0.46 - 0)p=0.047** | 0.03(-0.06 - 0.11)p=0.294 | -0.08(-0.18 - 0.02)p=0.059* | -0.15(-0.22 - -0.09)p=0.001** | 0.02(-0.02 - 0.06)p=0.167 | 0.01(-0.01 - 0.02)p=0.142 |
| Children deconfinementWeek 17-24, 2020 | -0.42(-0.63 - -0.25)p=0.001** | -0.02(-0.23 - 0.07)p=0.367 | -0.03(-0.13 - 0.08)p=0.266 | -0.01(-0.05 - 0.03)p=0.344 | -0.01(-0.04- 0.32)p=0.343 | -0.02(-0.04- -0.01)p=0.016** |
| Compulsory masksWeek 22-30, 2020 | -0.32(-0.51 - 0.14)p=0.003** | -0.02(-0.10 - 0.08)p=0.386 | -0.05(-0.14 - 0.05)p=0.163 | -0.02(-0.10 - 0.07)p=0.348 | -0.01(-0.05 - 0.03)p=0.345 | -0.02(-0.04 - 0.01)p=0.015** |
| Schools reopeningWeek 38-45, 2020 | -0.01(-0.21 - 0.17)p=0.437 | -0.08(-0.16 - 0.02)p=0.051* | 0.01(-0.08 - 0.11)p=0.373 | -0.01(-0.08 - 0.08)p=0.464 | -0.01(-0.45 - 0.03)p=0.368 | -0.01(-0.03 - 0.01)p=0.112 |
| End of compulsory masksWeek 16-25, 2022 | 0.01(-0.16 - 0.18)p=0.473 | 0.09(-0.01 - 0.22)p=0.080* | 0.03(-0.06 - 0.12)p=0.249 | -0.02(-0.09 - 0.06)p=0.315 | 0.03(0.01 - 0.06)p=0.033** | 0.01(-0.01 - 0.02)p=0.372 |
Average absolute effect (95% confidence interval)
The recent SARS-CoV-2 pandemic provided a unique opportunity to evaluate the impact of non-pharmaceutical interventions on limiting the spread of infections. This study expands on the current literature by examining the effects of confinement and strict social control measures on the spread of respiratory viruses, as well as the changes following the lifting of these measures. Unlike previous studies, this research employs deseasonalized time-series analysis to quantify the change in viral detection rates after each measure's implementation or removal.
Rhinovirus was the most frequently detected virus, followed by RSV and AdV. This is consistent with previous research highlighting the role of RV and RSV as major contributors to pediatric respiratory infections.10 In the pre-pandemic phase, RV and AdV exhibited a year-round virus pattern. In contrast, RSV, Influenza, and HMPV showed clearly epidemic periods during the winter months, as is typically described in temperate regions.11–13
With the emergence of the highly transmissible SARS-CoV-2,14 international authorities urged the implementation of strong isolation measures, previously used in earlier pandemics. For instance, during the H1N1 2009 influenza pandemic, social distancing, and early school closure effectively moderated the peak case incidence in some settings.15,16 During the lockdown, the most stringent of the NPI measures, there was a decrease in the positivity rate of all respiratory viruses, as reported in other European countries.11,12,17,18 Some studies documented a reduction in daily emergency room visits17 and hospital admissions due to respiratory tract infections coinciding with the introduction of confinement,17,19 when children and adults were practicing social distancing. Particularly, during this period, we observed a significant decrease in RV and HMPV detections, as well as a tendency for AdV detections in the short-term.
After the end of confinement for children, between the 17th and the 29th week of 2020, the trend for viral detection in them remained stable for all viruses. In the short-term analysis, we observed a significant decrease in RV and HMPV detections, as well as tendencies to statistically significant results in AdV and other detections. It should be noted that there was some overlap between children's deconfinement and confinement period, which may have led to delayed effects from the earlier measures observed during the subsequent period. However, the expected changes in trends following the release of children from confinement, which was a relaxation measure after a strict one, were not observed. This suggests that children's deconfinement had no short-term impact on the transmission of viruses. This pattern was also identified in other European countries, such as the United Kingdom,11 Finland,18 and in countries in the southern hemisphere, such as New Zealand.19
Interestingly, the trend graphics in the deseasonalized analysis showed that the detection rates for RSV, Influenza, and HMPV did not start to decline until after the mandatory mask requirement was implemented in the 23rd week of 2020. It was also associated with a short-term decrease in RV and other virus detections. As previously mentioned, the most stringent measure of the NPI was confinement. Therefore, a delayed effect of confinement on the circulation of these viruses may explain this finding. Strict public health restrictions can significantly reduce respiratory virus transmission, resulting in a decrease in childhood morbidity, including hospital and ICU admissions.20,21 In our environment, the use of face masks in adults could provide protection against viral infections in children under 6 years of age, who were not obliged to wear them. While systematic reviews have raised, some controversy about the effectiveness of face masks in protecting against viral infections:22,23 some articles in the literature support their use in controlling the source of infection.24,25 There is evidence that masks are effective in the community setting if they are used early.26 Low adherence to the implementation of social isolation measures within households may favour intrafamilial transmissibility. The study of Otomaru et al. analyzed intrafamilial transmission of RSV and found that most transmission events occurred in the week following exposure within the home.26,27
Our study, in line with others,18 found that the start of the 2020-21 school year did not lead to an immediate increase in viral detections in the short term. As the school year progressed, coinciding with increased interactions among children in a school setting and other relaxation of lockdown rules, there was a progressive increase in RV determinations, as described in other countries around the world.11,19,28,29 However, detection levels remained lower than before the pandemic. Lumley and Mansuy et al. reported a similar pattern of RV detection in their studies and noted an increased detection of AdV,11,30 which is consistent with our findings. Huang and Takashita et al. suggested that the increased incidence of RV could be due to the relaxation of isolation measures. Indeed, transmission is facilitated by factors such as cohabitation and the virological characteristics of the RV, a non-enveloped virus that is less susceptible to inactivation by hand washing or disinfectants and can survive on surfaces for extended periods.19,29 Haapanen et al. study showed that the incidence of RV in children increased during summer vacation, coinciding with the lifting of social restrictions, and before the reopening schools. This suggests that day care centers and schools might not be the main drivers of RV spread.18
Interestingly, the peak of RSV, HMPV, and Influenza was delayed, potentially due to the influence of NPI measures. In our region, RSV incidence remained low since the 20th week of 2021, like other European and Southern Hemisphere countries, until an inter-seasonal spike in RSV incidence during the summer of 202129,31–34 particularly among older children.35,36 This spike might be attributed to a lack of pre-existing immunity due to decreased exposure in the preceding years. The inter-seasonal spike could be attributable to increased social interaction due to the reopening of leisure centers or parents returning to work.11 In contrast, countries such as France and Iceland, which gradually eased restrictions between November 2020 and February 2021 while keeping schools open, experienced a seasonal spike in the winter of 2020/21, starting several weeks later than usual.35
Following the discontinuation of compulsory mask usage, we observed a short-term increase in Influenza detections, even though, the flu season had not finished when this decision was taken. This resurgence suggests that mask mandates helped to supress Influenza transmission. Additionally, a drop in the detection rates of RV and RSV was interrupted at that time, and detections slightly increased, but these changes were not statistically significant in the short-term interrupted analysis. The differential impact of mask usage on different viruses highlights the complexity of viral interactions and the need for multifaceted approaches to manage their transmission.
Viral interference seems to be another factor influencing the presence or absence of viruses in the community.13,37 A negative association between RSV and RV detection was observed in our study although the number of cases detected was low in absolute values.38 Achten et al. reported that negative association was consistently present across RSV seasons, calendar month, disease severity spectrum, and geographical regions.39 Traditionally, a competitive relationship has been described between RSV and Influenza. During the influenza H1N1 pandemic, the change in influenza activity was associated with a shift in seasonal RSV activity, suggesting viral interference between both viruses.40
This study has several limitations. Firstly, the findings are specific to a single center's population and should be understood within the local epidemiological context. Secondly, the study's focus on patients in the PICU and those with chronic comorbidities may lead to the underrepresentation of healthy children, potentially limiting the generalizability of the viral detection rates to the broader population. However, these groups are instrumental in effectively evaluating NPI measures. Despite the inclusion of high-risk outpatients with mild respiratory diseases, which does expand the representation and reflects the majority of infection types prevalent in the overall pediatric population, restricting testing to these populations resulted in a comparably small final sample size to the regional pediatric reference population. Finally, results were not stratified by patient age, yet statistically significant differences were observed in the initial descriptive analysis due to inclusion of respiratory samples from patients meeting criteria regardless of age.
ConclusionIn conclusion, this study highlights the significant impact of NPIs in reducing respiratory virus transmission among children, even when not tailored exclusively to preschool-aged children. Rhinovirus, RSV, HMPV, and Influenza appear particularly responsive to these interventions, both upon implementation and cessation. These findings enhance our understanding of respiratory virus transmission dynamics, offering insights into the potential effectiveness of NPIs in curbing their spread.
Funding detailsNo funding was received to perform this study.
Disclosure statementThe authors report there are no competing interests to declare.
Conflict of interestsThe authors state that they have no conflict of interests.