Duchenne Muscular Dystrophy (DMD) is the most common inherited muscle disease in children, with a prevalence ranging between 1.3 and 2.1 per 10,000 live male births.1 Acute Respiratory Failure (ARF) is a common complication in individuals with DMD and a primary cause of mortality, both in the teenage and adult years.2
Non-Invasive Ventilation (NIV) is a ventilatory technique for delivering pressurized gas to the lungs through a sealed mask that can be placed over the mouth, nose or the whole face; its utilization is recommended for the management of ARF across a variety of aetiologies. However, NIV application in exacerbated DMD patients is still controversial and not indicated by major guidelines.3, The aim of our study was to evaluate clinical status and outcomes in DMD patients being administered NIV for ARF.
We collected and reviewed all of the medical records of the DMD patients with ARF who were admitted to our adult 4 bed Respiratory ICU (RICU) at the University of Padua Medical Center between January 1, 2005 and December 31, 2019. Ethical approval was waived by the local Ethics Committee in view of the fact that the study was retrospective and not prepared according to a research project.
At the time of admission to the RICU, the clinical and physiologic parameters of these patients were consistent with ARF. In particular, each of them presented at least one of the following: (1) respiratory distress; (2) hypoxemia and/or hypercapnia; (3) acute respiratory acidosis.4 Patients who had received NIV as first-line ventilatory treatment were included in the study; those who had a tracheostomy tube in place or showed an immediate need for Endotracheal Intubation (ETI)5 were excluded.
All hospital charts were reviewed for patients’ baseline clinical, demographic and pulmonary function data after informed consent release forms were obtained. Clinical, laboratory and blood gas data at RICU admission were also recorded and analyzed.
Causes of ARF were classified as: upper respiratory tract infection (URTI); acute decompensated heart failure (ADHF); pneumonia; and other (e.g., pneumothorax, pulmonary thromboembolism, dysphagia with aspiration, gastroparesis/malnutrition, acute gastrointestinal distension, abuse of sedatives). The diagnosis of URTI was based on the presence of one or more of the following symptoms or signs: fever, throat irritation or sore throat, hoarseness6; the diagnoses of pneumonia and/or ADHF were based on major guidelines.7,8
The decision to begin NIV was usually made by the attending physician, according to the hospital internal protocol.9 NIV was usually delivered using a portable ventilator set in assisted pressure-control ventilation (APCV) mode. A commercial full-face mask was used when NIV was initiated and in some cases it was substituted by a nasal mask after the first hours of ventilation. In the event of NIV failure, the patients were shifted from NIV to Invasive Mechanical Ventilation (IMV) by ETI unless they had previously declared that they did not wish to be intubated. Mechanical In-Exsufflation (MI-E) was usually administered to those patients showing bronchial mucous encumbrance and oxyhemoglobin desaturation secondary to secretion retention, according to the hospital internal protocol.6
The patients were divided into 2 groups depending on their NIV response. “NIV success group” was made up of those individuals who avoided ETI, were discharged from RICU and survived for at least 48h after being transferred to the respiratory ward. “NIV failure group” was made up of those subjects who required ETI or died while on NIV support.
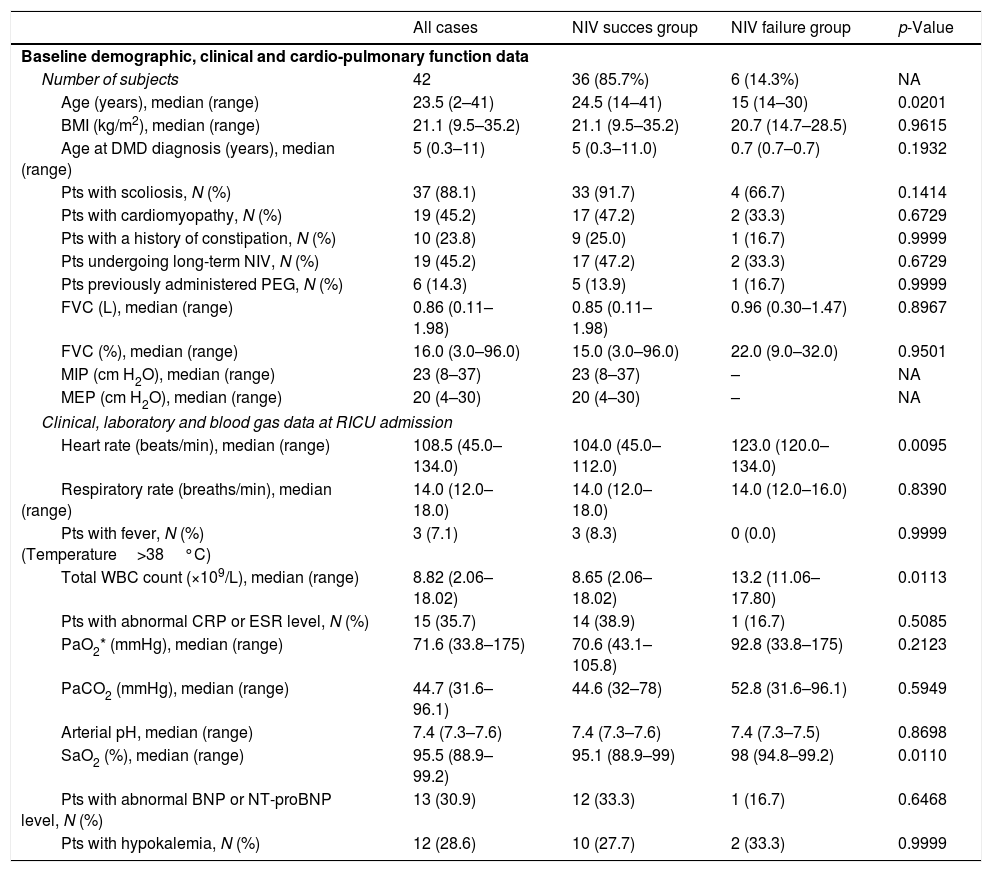
During the study period, 51 patients were admitted to our RICU for ARF with a primary diagnosis of DMD. Seven (13.7%) had a tracheostomy tube in place and were administered IMV via tracheal cannula, 2 (3.9%) were intubated at admission, while all the remaining 42 (82.3%), received NIV as the first-line ventilatory intervention and were considered eligible to participate in our retrospective study. No patient experienced repeated episodes of ARF. Of notice, RICU admissions were evenly distributed during the study period. Baseline demographic, clinical, pulmonary and cardiac function, of the patients are outlined in Table 1. Conditions associated with the onset of ARF in the patients studied included URTI (19 cases); ADHF (5 cases); pneumonia (4 cases); and other conditions (14 cases). The patients were administered NIV in APCV. The initial inspiratory pressure above PEEP was 12 (8–25) cmH2O. All subjects were fully cooperative during NIV and did not require any sedation. NIV was successful in 36 patients (85.7%), while unsuccessful in the remaining 6 (14.3%). The median time from NIV initiation to intubation was 3.5 (1–11) days. Among patients who failed NIV, the cause of ARF was dysphagia with aspiration in 3 cases; ADHF in 2 cases; and pneumonia in 1 case. Causes of NIV failure were failure to adequately correct hypercapnia and exhaustion (3 cases), persistent accumulation of bronchial secretions (2 cases), and severe hemodynamic instability (1 case). Two patients died following intubation while on IMV support; the remaining four required tracheostomy due to difficult weaning and underwent long-term invasive ventilation. No major NIV complication was observed, while minor complications occurred in 4 patients (nasal skin lesions in 3 cases and acute bowel distension in 1 case). All 36 patients successfully treated and 4 out of 6 who failed NIV were discharged alive from hospital. Patients’ characteristics at RICU admission are outlined in Table 1. The median survival of the 42 NIV patients after RICU admission was 67.1 (95%CI, 15.0 to 125.4) days. Survival was significantly reduced for patients who failed NIV, compared to those who succeeded [median survival time: 11.7 (95%CI, 0.0 to 131.0) vs 85.9 (95%CI, 24.9 to 125.4) days], with an HR of dying of 3.91 (95%CI, 1.10 to 13.93; p=0.0351). None of the covariates had a significant effect on NIV failure. However, patients who did not respond to NIV were younger than those who succeeded [15 (14–30) vs. 24.5 (14–41) yrs; p=0.0201]; moreover, patients’ characteristics at RICU admission showed greater HR values [123.0 (120.0–134.0) vs. 104.0 (45.0–112.0) b/min; p=0.0095], total WBC count [13.2 (11.06–17.80) vs. 8.65 (2.06–18.02); p=0.0113] and SaO2 [98 (94.8–99.2) vs. 95.1 (88.9–99); p=0.011] for failures, as opposed to successes (Table 1).
Patients’ baseline demographic, clinical, and cardio-pulmonary function characteristics, and clinical, laboratory and blood gas data at RICU admission.
| All cases | NIV succes group | NIV failure group | p-Value | |
|---|---|---|---|---|
| Baseline demographic, clinical and cardio-pulmonary function data | ||||
| Number of subjects | 42 | 36 (85.7%) | 6 (14.3%) | NA |
| Age (years), median (range) | 23.5 (2–41) | 24.5 (14–41) | 15 (14–30) | 0.0201 |
| BMI (kg/m2), median (range) | 21.1 (9.5–35.2) | 21.1 (9.5–35.2) | 20.7 (14.7–28.5) | 0.9615 |
| Age at DMD diagnosis (years), median (range) | 5 (0.3–11) | 5 (0.3–11.0) | 0.7 (0.7–0.7) | 0.1932 |
| Pts with scoliosis, N (%) | 37 (88.1) | 33 (91.7) | 4 (66.7) | 0.1414 |
| Pts with cardiomyopathy, N (%) | 19 (45.2) | 17 (47.2) | 2 (33.3) | 0.6729 |
| Pts with a history of constipation, N (%) | 10 (23.8) | 9 (25.0) | 1 (16.7) | 0.9999 |
| Pts undergoing long-term NIV, N (%) | 19 (45.2) | 17 (47.2) | 2 (33.3) | 0.6729 |
| Pts previously administered PEG, N (%) | 6 (14.3) | 5 (13.9) | 1 (16.7) | 0.9999 |
| FVC (L), median (range) | 0.86 (0.11–1.98) | 0.85 (0.11–1.98) | 0.96 (0.30–1.47) | 0.8967 |
| FVC (%), median (range) | 16.0 (3.0–96.0) | 15.0 (3.0–96.0) | 22.0 (9.0–32.0) | 0.9501 |
| MIP (cm H2O), median (range) | 23 (8–37) | 23 (8–37) | – | NA |
| MEP (cm H2O), median (range) | 20 (4–30) | 20 (4–30) | – | NA |
| Clinical, laboratory and blood gas data at RICU admission | ||||
| Heart rate (beats/min), median (range) | 108.5 (45.0–134.0) | 104.0 (45.0–112.0) | 123.0 (120.0–134.0) | 0.0095 |
| Respiratory rate (breaths/min), median (range) | 14.0 (12.0–18.0) | 14.0 (12.0–18.0) | 14.0 (12.0–16.0) | 0.8390 |
| Pts with fever, N (%) (Temperature>38°C) | 3 (7.1) | 3 (8.3) | 0 (0.0) | 0.9999 |
| Total WBC count (×109/L), median (range) | 8.82 (2.06–18.02) | 8.65 (2.06–18.02) | 13.2 (11.06–17.80) | 0.0113 |
| Pts with abnormal CRP or ESR level, N (%) | 15 (35.7) | 14 (38.9) | 1 (16.7) | 0.5085 |
| PaO2* (mmHg), median (range) | 71.6 (33.8–175) | 70.6 (43.1–105.8) | 92.8 (33.8–175) | 0.2123 |
| PaCO2 (mmHg), median (range) | 44.7 (31.6–96.1) | 44.6 (32–78) | 52.8 (31.6–96.1) | 0.5949 |
| Arterial pH, median (range) | 7.4 (7.3–7.6) | 7.4 (7.3–7.6) | 7.4 (7.3–7.5) | 0.8698 |
| SaO2 (%), median (range) | 95.5 (88.9–99.2) | 95.1 (88.9–99) | 98 (94.8–99.2) | 0.0110 |
| Pts with abnormal BNP or NT-proBNP level, N (%) | 13 (30.9) | 12 (33.3) | 1 (16.7) | 0.6468 |
| Pts with hypokalemia, N (%) | 12 (28.6) | 10 (27.7) | 2 (33.3) | 0.9999 |
BMI=Body Mass Index; BNP=Brain Natriuretic Peptide; CRP=C-Reactive Protein; DMD=Duchenne Muscular Dystrophy; ESR=Erythrocyte Sedimentation Rate; FVC=Forced Vital Capacity; MEP=Maximal Expiratory Pressure; MIP=Maximal Inspiratory Pressure; NA=not applicable; NIV=Non-Invasive Ventilation; NT-proBNP=N-terminal prohormone of Brain Natriuretic Peptide; PEG=Percutaneous gastrostomy; RICU=Respiratory Intensive Care Unit; SaO2=arterial oxygen saturation; WBC=White Blood Cell).
p-Values refer to differences between NIV success and NIV failure groups.
The use of NIV in DMD patients for ARF is still debated.10–12 In our study, the overall success rate of 86% corroborates the positive role of NIV in avoiding tracheal intubation. On our opinion, high success rate of NIV treatment in our patients can be explained by several factors, including the following. First, we used APCV, a mode of partial ventilatory support found to be comfortable and minimally affected by patient–ventilator asynchrony in individuals with ventilatory failure of neuromuscular origin.13 Second, we chose full-face mask as first choice. Indeed, nasal masks may be problematic in these patients because of weakness of masseter muscles, causing incomplete jaw closing and major mouth air leaks. Furthermore, patients with ARF are characterized by mouth-breathing making nasal masks ineffective. For these reasons, despite a potentially increased risk of gastric distension and emesis into the mask,14 a full face mask was preferred in our patients. Third, the combined use of MI-E was effective in clearing bronchial secretions in most patients who developed bronchial mucous encumbrance and consequent oxyhemoglobin desaturation, in keeping with previous data both in adults and children with NMD.15 Finally, it is worth remarking that our team of pulmonary physicians and nurses has been involved in NIV treatment of NMD patients with ARF for over 20 years.
Notwithstanding the low NIV failure rate, we believe that appropriate selection criteria for attempting NIV in this setting is a crucial unresolved issue. Unfortunately, we found none of the covariates associated with NIV failure. Noteworthy, patients who did not respond to NIV were younger than those who succeeded and reported dysphagia, weight loss and aspiration in 3 out of 6 cases. Swallowing disturbances resulting in increased risk of inhalation have been reported as a possible mechanism of ARF in boys with DMD16 and a common cause of NIV failure in NMD patients.9
Of interest, there was a trend toward higher PaO2, SaO2, and PaCO2 values in the NIV failure compared to NIV success group. We hypothesize that delivery of excessively high amounts of supplemental oxygen during transfer to our RICU might have increased the risk of NIV failure, by depressing spontaneous ventilation. Indeed, oxygen therapy has been shown to reduce the drive to breathe and lead to CO2 retention in DMD patients during respiratory exacerbations.17
Our study has important limitations, being single-centered, retrospective and observational. It is important to remind, however, that prospective randomized trials in this patient population are unlikely to be performed because of the limited number of patients affected with DMD and of possible ethical issues. The study's long time span is also a potential weakness. Worth mentioning, however, we did not make significant changes in pharmacologic therapy and/or supportive care during the study period.
Despite these major limitations, our study indicates NIV to be a viable option in DMD patients showing signs of ARF as it can effectively reverse ARF in the majority of patients, with the caveat that its use should be extremely careful in younger patients with dysphagia.