Evidence for the use of non-anesthesiologist-administered propofol for sedation during flexible bronchoscopy is scarce. The main objective of this study was to determine whether non-anesthesiologist-administered propofol balanced sedation was related to higher transcutaneous CO2 pressure compared with current guideline-based sedation (combination midazolam and opioid). Secondary outcomes were post-procedural recuperation time, patient satisfaction and frequency of adverse events.
MethodsIn this randomized controlled trial we included data from outpatients aged 18 years or older with an indication for flexible bronchoscopy in a university hospital in northern Mexico.
ResultsNinety-one patients were included: 42 in the midazolam group and 49 in the propofol group. During 60min of transcutaneous capnometry monitoring, mean transcutaneous CO2 pressure values did not differ significantly between groups (43.6 [7.5] vs. 45.6 [9.6]mmHg, P=.281). Propofol was related with a high Aldrete score at 5, 10, and 15min after flexible bronchoscopy (9 [IQR 6-10] vs. 10 [9,10], P=.006; 9 [8–10] vs. 10 [IQR 10–10], P<.001 and 10 [IQR 9–10] vs. 10 [10], respectively) and with high patient satisfaction on a visual analogue scale of 1 (not satisfied) to 10 (very satisfied) (8.41 [1.25] vs. 8.97 [0.98], P=.03). Frequency of adverse events was similar among groups (30.9% vs. 22.4%, P=.47).
ConclusionCompared with guideline-recommended sedation, non-anesthesiologist-administered propofol balanced sedation is not associated with higher transcutaneous CO2 pressure or with more frequent adverse effects. Propofol use is associated with faster sedation recovery and with high patient satisfaction.
Clinical trial RegistrationNCT02820051.
Las pruebas disponibles del uso de propofol administrado por no anestesiólogos para la sedación durante la broncoscopia flexible son escasas. El objetivo principal del estudio fue determinar si la sedación equilibrada con propofol administrado por no anestesiólogos estaba relacionada con valores más altos de presión de CO2 transcutánea, en comparación con la sedación según las pautas (una combinación de midazolam y un opiáceo). Las variables secundarias fueron el tiempo de recuperación después del procedimiento, el grado de satisfacción del paciente y la frecuencia de observación de efectos adversos.
MétodosEn este ensayo controlado y aleatorizado se incluyeron datos de pacientes ambulatorios mayores de 18 años con indicación de broncoscopia flexible en un hospital universitario del norte de México.
ResultadosSe incluyeron 91 pacientes: 42 en el grupo de midazolam y 49 en el grupo de propofol. Durante los 60min de monitorización de la capnometría transcutánea, no hubo diferencias estadísticamente significativas entre grupos en los valores medios de presión de CO2 transcutánea (43,6 [5,7] vs. 45,6 [6,9]mm Hg, p=0,281). El propofol se asoció con puntuaciones de Aldrete altas a los 5, 10 y 15min después de la broncoscopia flexible (9 [IQR: 6-10] vs. 10 [9,10], p=0,006; 9 [8-10] vs. 10 [IQR 10-10], p<0,001 y 10 [IQR 9-10] vs. 10 [10] puntos, respectivamente) y con un alto grado de satisfacción de los pacientes en una escala visual de 1 (poco satisfecho) a 10 (muy satisfecho) (8,41 [1,25] vs. 8,97 [0,98], p=0,03). No hubo diferencias en la frecuencia de efectos adversos (30,9 vs. 22,4%, p=0,47).
ConclusiónEn comparación con la pauta de sedación recomendada, la sedación equilibrada con propofol administrado por no anestesiólogos no se asocia con valores más altos de presión de CO2 transcutánea ni con mayor frecuencia de efectos adversos. El uso del propofol se asocia con una recuperación de la sedación más rápida y con un mayor grado de satisfacción del paciente.
Número de registro del ensayo clínicoNCT02820051.
Flexible bronchoscopy (FB) is a fundamental procedure in respiratory medicine. Although FB can be performed safely without sedation, 80% of patients prefer sedation during the procedure.1 Currently, sedation and analgesia are considered the standard of care in patients without contraindication for its administration.2 Although midazolam is recommended for sedation in most guidelines,2–5 propofol has gained popularity, mainly due to its short recovery time. However, evidence for propofol use during FB is scarce, especially when it is not administered by ananesthesiologist.
Respiratory depression is a major concern during respiratory endoscopy. While SO2 monitoring is performed in all patients undergoing FB, CO2 monitoring is less common. For many years, CO2 measurement required an arterial blood sample; however, it can now be monitored with transcutaneous capnometry. The transcutaneous pressure of CO2(TcPCO2) is well correlated with PaCO2,6 and several authors have used transcutaneous capnometry in patients undergoing different sedation protocols for endoscopic procedures7–9; however, there is little evidence on TcPCO2 changes in patients sedated with midazolam or propofol who have received concomitant intravenous opioids (e.g., balanced sedation).
In this randomized controlled trial, we evaluated ventilation response measured by transcutaneous capnometry in adult patients undergoing ambulatory FB who received non-anesthesiologist-administered propofol (NAAP) balanced sedation, and compared it with guideline-recommended sedation consisting of combination midazolam and opioid. Our primary outcome was to assess difference between groups in TcPCO2 values during and after FB. We hypothesized that TcPCO2 values would not be higher in patients who received NAAP balanced sedation. Secondary outcomes were procedural recovery time measured using the Aldrete scale, patient satisfaction, and frequency of adverse events.
Materials and MethodsPatients and ProceduresBetween February and July 2014, we prospectively included ambulatory patients >18 years of age with an indication for FB. Bronchoscopy procedures were performed by respiratory and critical care medicine residents under the supervision of an associate professor in a tertiary-referral university hospital in northern Mexico. Patients with tracheostomy, known allergy to study drugs, psychiatric illness, pregnancy, American Society of Anesthesiologist physical status class IV or V, or capnometry sensor dysfunction were excluded.
Patients were assigned by block randomization to receive midazolam or propofol. In the midazolam group the initial dose was 0.05mg/kg, and in the propofol group the starting dose was 0.1mg/kg. Additional doses of the corresponding drug (2mg of midazolam or 10mg of propofol) were permitted to obtain a score of 3 to 4 on the observer's assessment of alertness/sedation scale. All patients received nalbuphine at a starting dose of 2mg with additional doses of 1mg if necessary. Lidocaine spray was applied to the pharynx and also to the nasal mucosa when bronchoscope was inserted through a nostril. Endobronchial topical lidocaine was applied using the spray-as-you-go technique, at a maximum dose of 7mg/kg. Sedation and analgesia were prescribed by the resident responsible for conducting FB and administered by an auxiliary nurse without the support of an anesthesiology specialist.
TcPCO2 measurement was carried out with the SenTec digital monitoring system (Artemis Medical, Kent, London) by applying a Stow–Severinghaus (V-Sign sensor) type sensor in the ear lobe. We monitored TcPCO2 for 1h from the start of FB, and recorded TcPCO2 values every 5min for the first 20min and every 10min up to 60min. All patients received supplementary oxygen and were monitored with periodic non-invasive blood pressure measurements, continuous electrocardiogram, and SO2 surveillance.
Residual sedation was measured on the Aldrete scale at 5, 10, and 15min after FB. At the time of discharge from the bronchoscopy suite, satisfaction was assessed using a visual analog scale of 1 (not satisfied) to 10 (very satisfied). One investigator, blinded to the patient's study group, recorded all procedural data. The bronchoscopist was blinded to TcPCO2 values.
Sample Size and Statistical AnalysisThe sample was calculated for alpha 0.05, beta 0.20, standard deviation of 7.3,8 minimum TcPCO2 difference to detect of 5mmHg, estimated loss to follow-up of 0.20, and two-tailed analysis. According to the above, the sample size was 42 patients per group.
We tested normal distribution with the Kolmogorov–Smirnov test. Data are shown as means and standard deviation for variables with normal distribution, and as median and interquartile range for non-normal variables. We used the t-test, the Mann–Whitney U-test, ANOVA, or chi-square as indicated. We defined a statistically significant difference as a P value <.05. The analysis was performed using SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL).
Ethical ConsiderationsAll participating physicians received formal training in procedural sedation with propofol from professors of the UANL University Hospital's Anesthesiology Department before the start of the trial. All residents were certified by the American Heart Association in Advanced Cardiopulmonary Life Support. The study was approved by the narcotics and ethics committees of the UANL University Hospital (registration number NM13-009). All patients provided informed consent.
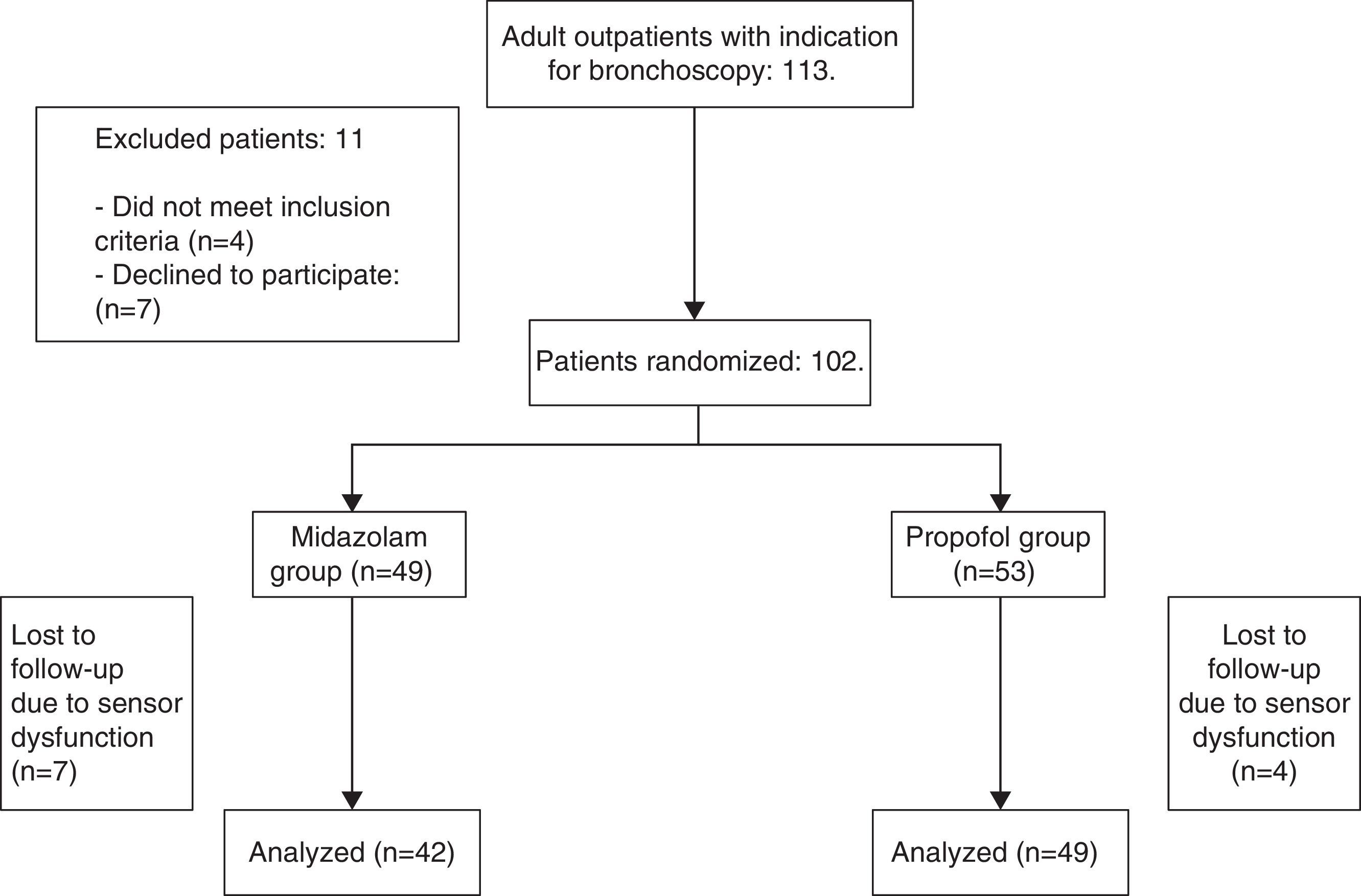
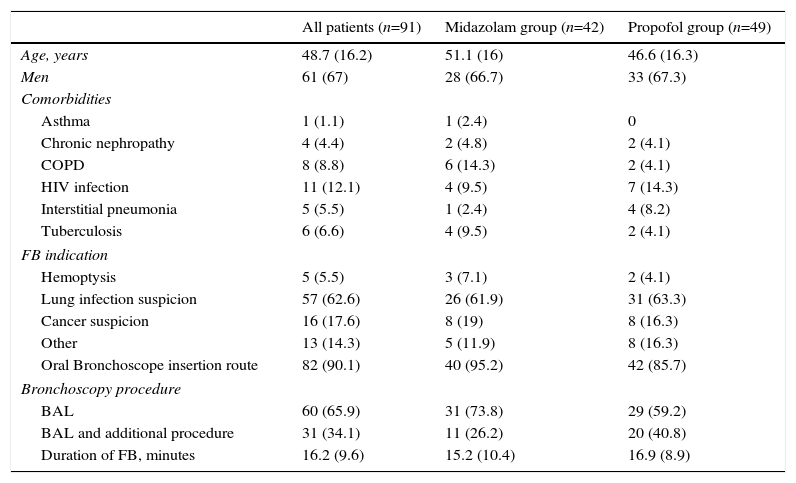
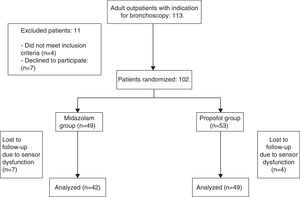
ResultsWe included 91 patients, 42 (46.1%) in the midazolam group and 49 (53.8%) in the propofol group. The process of inclusion and randomization is shown in Fig. 1. There were no statistically significant differences between groups with respect to age, sex, comorbidities, indication for endoscopic study, bronchoscope insertion route, additional bronchoscopy procedures, or duration of FB (Table 1).
Demographic and Clinical Data. Data are Shown as Number and Percentage or Mean and Standard Deviation, as required.
| All patients (n=91) | Midazolam group (n=42) | Propofol group (n=49) | |
|---|---|---|---|
| Age, years | 48.7 (16.2) | 51.1 (16) | 46.6 (16.3) |
| Men | 61 (67) | 28 (66.7) | 33 (67.3) |
| Comorbidities | |||
| Asthma | 1 (1.1) | 1 (2.4) | 0 |
| Chronic nephropathy | 4 (4.4) | 2 (4.8) | 2 (4.1) |
| COPD | 8 (8.8) | 6 (14.3) | 2 (4.1) |
| HIV infection | 11 (12.1) | 4 (9.5) | 7 (14.3) |
| Interstitial pneumonia | 5 (5.5) | 1 (2.4) | 4 (8.2) |
| Tuberculosis | 6 (6.6) | 4 (9.5) | 2 (4.1) |
| FB indication | |||
| Hemoptysis | 5 (5.5) | 3 (7.1) | 2 (4.1) |
| Lung infection suspicion | 57 (62.6) | 26 (61.9) | 31 (63.3) |
| Cancer suspicion | 16 (17.6) | 8 (19) | 8 (16.3) |
| Other | 13 (14.3) | 5 (11.9) | 8 (16.3) |
| Oral Bronchoscope insertion route | 82 (90.1) | 40 (95.2) | 42 (85.7) |
| Bronchoscopy procedure | |||
| BAL | 60 (65.9) | 31 (73.8) | 29 (59.2) |
| BAL and additional procedure | 31 (34.1) | 11 (26.2) | 20 (40.8) |
| Duration of FB, minutes | 16.2 (9.6) | 15.2 (10.4) | 16.9 (8.9) |
BAL: bronchoalveolar lavage; COPD: Chronic Obstructive Lung Disease; FB: flexible bronchoscopy; HIV: Human Inmunodeficiency Virus.
There were no statistically significant differences between groups.
The mean dose of midazolam and propofol was 5.5 (2.2) and 122 (67.7) mg, respectively. The dose of nalbuphine was similar between groups (3 [IQR 2–4] vs. 2 [IQR 2–4] mg, P=.898).
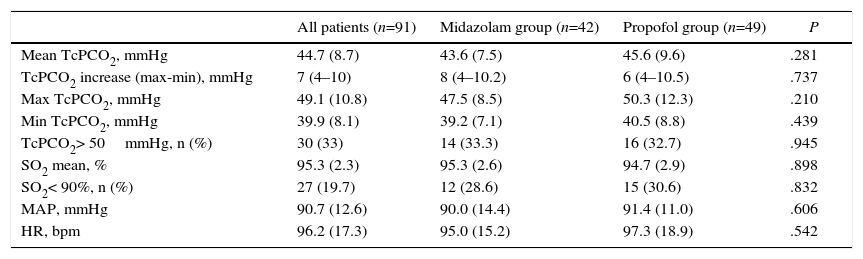
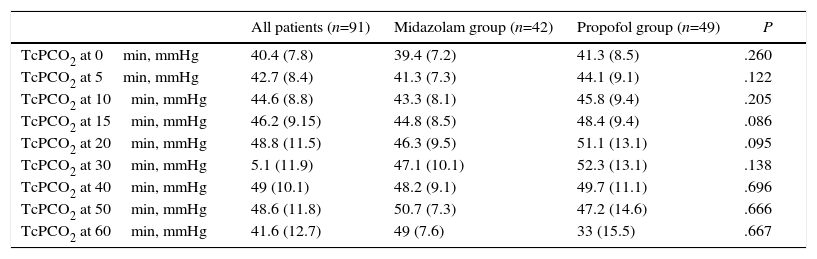
There were no statistically significant differences between groups in mean TcPCO2, minimum or maximum TcPCO2, increase in TcPCO2 (maximum TcPCO2-minimum TcPCO2), patients with TcPCO2 above 50mmHg, mean SO2, episodes of SO2 less than 90%, or mean arterial pressure or heart rate (Table 2). TcPCO2 did not differ at any time during or after FB (Table 3).
Transcutaneous PCO2 Pressure and Vital Signs During Flexible Bronchoscopy by Sedation Group.
| All patients (n=91) | Midazolam group (n=42) | Propofol group (n=49) | P | |
|---|---|---|---|---|
| Mean TcPCO2, mmHg | 44.7 (8.7) | 43.6 (7.5) | 45.6 (9.6) | .281 |
| TcPCO2 increase (max-min), mmHg | 7 (4–10) | 8 (4–10.2) | 6 (4–10.5) | .737 |
| Max TcPCO2, mmHg | 49.1 (10.8) | 47.5 (8.5) | 50.3 (12.3) | .210 |
| Min TcPCO2, mmHg | 39.9 (8.1) | 39.2 (7.1) | 40.5 (8.8) | .439 |
| TcPCO2> 50mmHg, n (%) | 30 (33) | 14 (33.3) | 16 (32.7) | .945 |
| SO2 mean, % | 95.3 (2.3) | 95.3 (2.6) | 94.7 (2.9) | .898 |
| SO2< 90%, n (%) | 27 (19.7) | 12 (28.6) | 15 (30.6) | .832 |
| MAP, mmHg | 90.7 (12.6) | 90.0 (14.4) | 91.4 (11.0) | .606 |
| HR, bpm | 96.2 (17.3) | 95.0 (15.2) | 97.3 (18.9) | .542 |
bpm: beats per minute; HR: heart rate; MAP: mean arterial pressure; SO2: O2 saturation; TcPCO2: transcutaneous PCO2 pressure. Data are shown as mean and standard deviation or median and interquartile range, as required.
Transcutaneous CO2 Pressure at Different Time Intervals.
| All patients (n=91) | Midazolam group (n=42) | Propofol group (n=49) | P | |
|---|---|---|---|---|
| TcPCO2 at 0min, mmHg | 40.4 (7.8) | 39.4 (7.2) | 41.3 (8.5) | .260 |
| TcPCO2 at 5min, mmHg | 42.7 (8.4) | 41.3 (7.3) | 44.1 (9.1) | .122 |
| TcPCO2 at 10min, mmHg | 44.6 (8.8) | 43.3 (8.1) | 45.8 (9.4) | .205 |
| TcPCO2 at 15min, mmHg | 46.2 (9.15) | 44.8 (8.5) | 48.4 (9.4) | .086 |
| TcPCO2 at 20min, mmHg | 48.8 (11.5) | 46.3 (9.5) | 51.1 (13.1) | .095 |
| TcPCO2 at 30min, mmHg | 5.1 (11.9) | 47.1 (10.1) | 52.3 (13.1) | .138 |
| TcPCO2 at 40min, mmHg | 49 (10.1) | 48.2 (9.1) | 49.7 (11.1) | .696 |
| TcPCO2 at 50min, mmHg | 48.6 (11.8) | 50.7 (7.3) | 47.2 (14.6) | .666 |
| TcPCO2 at 60min, mmHg | 41.6 (12.7) | 49 (7.6) | 33 (15.5) | .667 |
Data are shown as mean and standard deviation. TcPCO2: transcutaneous CO2 pressure.
Duration of FB was significantly longer in the 31 patients in whom procedures additional to bronchoalveolar lavage were performed (24.4 [5.8] vs. 14.8 [7.6] min, P<.001). In these patients, TcPCO2 levels (43.62 [7.43] vs. 45.61 [9.56] mm Hg, P=.27), maximum TcPCO2 (47.50 [8.46] vs. 50.35 [12.15] mm Hg, P=.20) and TcPCO2 increase (8.31 [5.59] vs. 9.84 [8.17] mm Hg, P=.30) did not differ between groups.
There were no statistically significant differences in mean TcPCO2 between patients sedated by residents in their first, second, or third year of residency (44.81 [8.76], 42.47 [7.91] and 49.45 [8.88] mmHg, respectively; P=.091).
The most common side effect during the procedure was bronchospasm, in 15 (16.4%) patients; there were no statistically significant differences between groups (8 [19%] vs. 7 [14.2%] patients, P=.540). Other complications were respiratory depression, in 2 (2.2%) patients (1 [2.4%] vs. 1 [2%] patient, P=1.0), and hypotension corrected after intravenous fluids, in 7 (7.7%) patients (4 [9.5%] vs. 3 [6.1%] patients, P=.699). Composite adverse effects were similar between groups (13 [30.9%] vs. 11 [22.4%] events, P=.44). No adverse effects led to the suspension of FB or resulted in hospitalization. Neither phlebitis nor major arrhythmia were observed in any patient.
The propofol group reported a higher Aldrete residual sedation score at 5, 10 and 30min after completion of FB (9 [IQR 6–10] vs. 10 [9,10], P=.006; 9 [8–10] vs. 10 [IQR 10–10], P<.001 and 10 [IQR 9–10] vs. 10 [10] points, P=.005, respectively). A larger proportion of patients in the propofol group reported a score of 9 or more at 5, 10, and 15min (24 [57.1%], 29 [69%] and 33 [78.5%] vs. 42 [85.7%], 47 [95.9%] and 48 [97.9%] respectively, P<.05 for all comparisons).
Satisfaction with the procedure was higher in the propofol group (8.41 [1.25] vs. 8.97 [0.98], P=.03).
DiscussionNon-anesthesiologist-administration of propofol is controversial. However, its use in the field of digestive endoscopy has increased,10 and the necessity of an attending anesthesiologist during the procedure has been questioned due to the elevated cost this involves.11 Anesthesiologists, meanwhile, are concerned that adverse effects will increase if sedation is not administered by a specialist.12 In Europe, NAAP during digestive endoscopy is regulated by the joint guidelines of the European Society of Anesthesiology in conjunction with the European Society of Gastrointestinal Endoscopy and the European Society of Gastroenterology and Endoscopy Nurses and Associates.13 However, this document is far from being a solution to the NAAP controversy, as at least 21 medical societies in Europe do not agree with those recommendations.14 In pulmonary medicine, evidence on NAAP is still limited, and to the best of our knowledge there are no specific guidelines for the use of NAAP during FB. Bosslet et al. reported their experience in a university hospital where NAAP guided by trained nurses was performed routinely for several years. In that setting, NAAP was feasible and safe in bronchoscopy procedures.15
In this randomized controlled trial, we compared TcPCO2 values in patients undergoing ambulatory FB who received NAAP balanced sedation using the current guideline-recommended protocol based on a benzodiazepine plus an opioid. We found no differences between groups in TcPCO2 levels during or after the procedure. In addition, oxygenation and vital signs did not differ significantly. To the best of our knowledge, this is the first study to investigate TcPCO2 changes during sedation administered by respiratory medicine residents in patients who also received opioids.
A major argument for benzodiazepines use preference is the availability of an antidote to revert excessive sedation leading to respiratory depression.3,4 However, our results are supported by other investigators who also suggest that propofol is at least as safe as midazolam with regard to ventilatory response. Yldzdas et al. compared children sedated using different protocols during digestive endoscopy (propofol, ketamine, and midazolam with and without fentanyl or ketamine), and found no differences in TcPCO2 levels before or after the procedure.16 Camri et al. found high TcPCO2 levels at 5 and 10min after FB in patients sedated with midazolam and alfentanil in comparison with those who received propofol as monotherapy.8 More recently, Heuss et al. found no differences in TcPCO2 between patients who received midazolam or propofol for colonoscopy.9
The dose of midazolam and propofol in the present study was lower than that used in protocols without concomitant opioid analgesia7,8,17,18 and is concordant with studies in which opioid use was permitted.9,19 The practice of adding opioids to propofol is not without its detractors; Yoon et al. comparing alfentanil plus propofol vs. propofol alone, found no benefit in terms of cough frequency or patient and bronchoscopist satisfaction.20 Stolz et al. found no satisfaction differences in patients sedated with midazolam and hydrocodone vs. propofol without an opioid; however, in their study the propofol group presented higher cough frequency.18 Finally, Clark et al. noted that propofol was better tolerated than midazolam during FB without transbronchial biopsy; they did not use opioids in any group.21 In our opinion, administration of opioids during FB sedation reduces sedation requirements. In our trial, we found no evidence of an increased incidence of hypoventilation among patients who received combination propofol plus opioid.
To the best of our knowledge, there is no firm evidence linking a particular FB sedation protocol with a higher risk of complications. In the first randomized controlled trial of midazolam and opioid vs. propofol, Stolz et al. did not find a higher complication frequency between groups; this is also true for patients with chronic obstructive pulmonary disease.18 Propofol has been related with a lower incidence of hypertension and tachycardia.17 However, in our trial we did not observe significant differences in this regard. Oztruk et al. observed an increased frequency of major arrhythmias during FB in patients who received midazolam or propofol (8% and 12%, respectively).17 We did not observe major arrhythmia episodes in our study, a finding that is consistent with other authors.22,23
Recovery time after procedural sedation is an issue to consider. In the present study, patients in the propofol group had a higher Aldrete post-anesthetic recovery score. This is consistent with findings reported in the literature. A faster recovery time is one of the less controversial benefits of propofol sedation, and has even been documented in geriatric patients with hypoalbuminemia receiving sedation due to regional anesthesia for orthopedic surgery.24 Propofolis also superior to benzodiazepines in improving cognitive recovery. Despite the subjective feeling of alertness, cognitive deficit is present up to 3.5h after midazolam administration, even when patients had received flumazenil for sedation reversion.25 In contrast, patients sedated with propofol show faster improvement in cognition response.26 We did not perform a formal cognitive evaluation; however, a significantly greater proportion of patients scored at least 9 points on the Aldrete scale at 5, 15, and 30min after propofol sedation, indicating faster global recovery after the procedure. It is important to mention that a higher total Aldrete score was mainly due to higher motor activity and conscience domain scores, while respiration and circulation domain scores did not differ between groups.
The economic impact of FB sedation is also worth examining. As far as we know, no formal cost analysis comparing propofol with midazolam has been published; however, at least one study found the cost of propofol sedation protocol to be higher.18 The higher cost of propofol must be compensated by potential savings in resources, i.e., faster post-procedural recuperation time and no anesthesiologist. Finally, we eliminated 11 (10.7%) patients from the analysis due to TcPCO2 sensor problems. This proportion of technical failures was higher than the 7.2% described by Heuss et al. in 2012.9
This study has several limitations: (1) it was not blinded. Despite the fact that the investigator recording the information was blinded to the specific drug used during sedation, we cannot rule out bias caused by the bronchoscopist's knowledge of the study group arm. (2) All patients were ambulatory, with low or intermediate anesthetic risk. In accordance with current recommendations, NAAP is not indicated in high-risk patients. (3) As oral bronchoscopy insertion is associated with faster vocal cord visualization, less use of lidocaine, and no insertion failure,27 this is the route of choice for FBs in our center. (4) In our bronchoscopy service, nalbuphine is the only opioid available, therefore, our results cannot not be extrapolated to other opioids. (5) All our residents were board-certified specialists in internal medicine and, as part of the Pulmonary and Critical Care sub-specialization program, had been trained in propofol use; consequently, when health specialists with other educational profiles perform NAAP, the outcomes might be different. (6) Finally, as in normal clinical practice, the role of the supervising physician during FB was merely to monitor correct execution of the procedure and provide support in case of complications that cannot be handled by residents. He was not required to intervene in any of the 91 endoscopic studies; however, the supervised environment of a university hospital can increase the safety of NAAP.
In conclusion, the results of this randomized controlled trial suggest that compared with current guideline-based recommendations, NAAP balanced sedation is not related to higher TcPCO2 levels or a significant incidence of adverse events during or after FB when administered by pulmonary medicine and critical care residents. Propofol also seems to be associated with faster post-sedation recovery and greater patient satisfaction. Additional randomized trials, preferably double-blinded studies, are required to assess the efficacy and safety of this sedation strategy.
FundingThe equipment and drugs used in this study were provided by the Department of Pulmonary and Critical Care Medicine of the “Dr. José E. González” University Hospital. This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
AuthorshipJEG had full access to all the data in the study, takes responsibility for the integrity of the data and the accuracy of the data analysis, particularly any adverse effects, and assumes full responsibility for the integrity of the submission as a whole, from inception to published article. CA, JEG, JT, RM contributed substantially to the study design, data analysis and interpretation, and the drafting of the manuscript.
Conflict of InterestThe authors declare that they have no conflict of interest.
We thank Belia I. Garduño-Chávez for her administrative support in the use of propofol in the bronchoscopy suite. Also, Erik J. Rendón-Ramírez, Alexis S. Herrera-Guerra, Sergio S. López-Salazar and Diego Hernández-Velázquez for their help in coordinating and performing bronchoscopies; and Roxana Saldaña-Vázquez, for her help in reviewing the manuscript.