The analysis of exhaled breath condensate (EBC) is a non-invasive technique that enables the determination of several volatile and nonvolatile substances produced in the respiratory tract, whose measurement may be useful for the diagnosis and monitoring of several respiratory diseases.
ObjectiveThe aim of this study was to produce a low-cost reusable device in order to sample exhaled breath condensate in healthy adult volunteers, and to determine the concentration of nitric oxide in the sample collected.
Material and methodsThe apparatus was made with a U-shaped tube of borosilicate glass. The tube was placed in a container with ice, and unidirectional respiratory valves were fitted to the distal end. Afterwards, nitric oxide was measured in the exhaled breath condensate (EBC) by chemiluminescence.
ResultsThe total cost of the device was $120.20. EBC samples were obtained from 116 volunteers of both sexes, aged between 20 and 70. The mean volume of exhaled breath condensate collected during 10minutes was 1.0±0.6mL, and the mean level of nitric oxide was 12.99±14.38μM (median 8.72μM). There was no correlation between the nitric oxide levels in the exhaled breath condensate and age or gender.
ConclusionWe demonstrate that it is possible to fabricate a low-cost, efficient, reusable device in order to collect and determine nitric oxide levels in EBC. We have identified no correlation between the nitric oxide levels present in the EBC obtained with this method with either age or sex.
El análisis del condensado de aire espirado (o exhalado) es una técnica no cruenta que permite determinar diversas sustancias volátiles y no volátiles producidas en las vías respiratorias, y cuya medición puede ser útil para el diagnóstico y la supervisión de diversas enfermedades respiratorias.
ObjetivosEl objetivo del presente estudio fue producir un dispositivo reutilizable, de bajo coste, para obtener muestras del condensado de aire espirado en individuos voluntarios sanos y determinar la concentración de óxido nítrico en la muestra recolectada.
Material y métodosEl dispositivo se fabricó con un tubo de vidrio de borosilicato en forma de U. El tubo se introdujo en un recipiente con hielo y en el extremo distal se adaptaron válvulas respiratorias unidireccionales. Acto seguido, se determinó el óxido nítrico en el condensado de aire espirado (CAE) mediante un método de quimioluminiscencia.
ResultadosEl coste total del dispositivo fue de 120,20 dólares. Las muestras de CAE se obtuvieron a partir de 116 individuos de ambos sexos, de 20-70 años de edad. El volumen medio del condensado de aire espirado obtenido durante 10min fue de 1,0±0,6ml y el nivel medio del óxido nítrico fue de 12,99±14,38μM (mediana, 8,72μM). No hubo correlación entre los niveles de óxido nítrico en el condensado de aire espirado y la edad o el sexo.
ConclusiónDemostramos que es posible fabricar un dispositivo de bajo coste, eficiente y reutilizable, para obtener y determinar los niveles de óxido nítrico en el CAE. Tampoco hemos identificado una correlación entre los valores de óxido nítrico presente en el CAE obtenido a través de este método, la edad y el sexo.
The analysis of biomarkers in exhaled breath condensate (EBC) has been proposed as a non-invasive technique for researching several inflammatory and non-inflammatory respiratory diseases and as a tool to follow up these diseases.1–4 Among the biomarkers of interest, nitric oxide (NO), a free radical that participates in a wide variety of pertinent biochemical reactions, has emerged as an important biological marker.5–8 Exhaled NO has been intensely researched and seems to be a useful marker in asthma9 and chronic obstructive pulmonary disease (COPD).10 Its values have also been demonstrated to be higher in lung cancer patients11 and can be correlated with clinical lung injury scores.12
Despite the fact that devices for collecting EBC are commercially available, these devices are disposable and in many countries they are not readily available, especially in developing countries where the import costs limit their systematic use in clinical practice. Furthermore, hardly any information is available about the NO content in the EBC of healthy individuals. Some studies provide the values of the mean concentration in healthy individuals (Table 1), and the variability of the values may be due to the different types of devices used.
Values From Published Studies Analyzing Nitric Oxide (NO) Concentrations.
| Author | Year | Place | n | Age, Mean Values | Method | Mean Values of NOx, μM |
| Ganas et al.25 | 2001 | Greece | 10 | 23 (4) | Hand-made | 0.63 |
| Franklin et al.26 | 2006 | Australia | 25 | 23–55 | Hand-made | 1.82 |
| Dressel et al.27 | 2010 | Germany | 26 | 31 (8.3) | EcoScreen® | 3.73 |
| Liu et al.23 | 2007 | Australia | 15 | 65 (9.38) | Hand-made | 9.89 |
| Hoffmeyer et al.24 | 2007 | Germany | 16 | 26–62 | EcoScreen® | 7.11 |
| Ojoo et al.22 | 2005 | London | 15 | 39 (10) | Hand-made | 11.4 |
For this reason, in order to resolve these difficulties, we decided to fabricate the instrument proposed by Horváth et al.13 at our institution with a few modifications in order to obtain and analyze NO in the EBC of a cohort of healthy adults.
MethodsThe present study was approved by the research committee of our institution (HCRP protocol number 4167/2009). All the experiments were done in accordance with ethical principles of human medical research (World Medical Association, Helsinki Declaration).
VolunteersWe recruited 116 healthy volunteers, both male and female, aged from 20 to 70. They were assigned to age groups that were stratified by 10-year increments. Excluded from the study were any volunteers who had a history of either smoking, drug addiction, lung disease and/or chronic systemic disease, including allergies, acute respiratory infection within the last 30 days, recent and/or constant exposure to formaldehyde and ozone, and the use of certain drugs, such as angiotensin-converting enzyme inhibitors, phosphodiesterase inhibitors, NO donor drugs (nitroprusside), steroids, oxymetazoline and non-steroidal anti-inflammatory drugs. We also excluded any individual who had eaten within 2h before the collection of EBC or who had done any strenuous physical exercise, consumed alcohol or taken any medicine in the previous 24h.
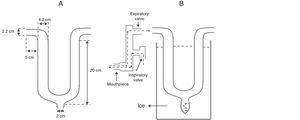
Device for Collecting Exhaled Breath CondensateIn short, the device was made with a U-shaped glass tube (borosilicate glass, Duran® Schott-Rohrglas, Mitterteich, Germany) that was 18mm thick. At the lower distal end of the glass tube, an opening was made which was connected to a polypropylene tube (1.5ml capacity, Axygen Inc., California, USA) for collecting EBC. At the other open end of the tube, unidirectional respiratory valves (K Takaoka, São Paulo, Brazil) were connected, which separated the inhaled (room) air from the exhaled air in order to direct all the expired air through the tube. A digital pneumotachometer (Ventronic II, DHD Healthcare, New York, USA) was connected to the opposite end. Fig. 1 shows the design and the dimensions of the glass tube as well as the assembly for collecting EBC.
After each use, the Teflon and silicone pieces were cleaned with soap and water and were placed in a container with peracetic acid (Anioxyde 1000, Anios, Pavê Du Moulin, Lille, France) at a concentration of 1471 parts per million for 30min. Right afterwards, they were rinsed in water and dried. The glass tube was rinsed with abundant distilled water (Milli-q) and sterilized by autoclave (HI VAC, Baumer, São Paulo, Brazil).
Obtaining Exhaled Breath CondensateInstructions were given to all the volunteers to explain the necessary steps, which were repeated with a similar device before obtaining EBC. Just before the EBC maneuver, the individuals rinsed out their mouths with distilled water. Using a nose clip, the individuals were instructed to breathe calmly through the mouth of the device for 10min. During the entire procedure, the entire glass tube as well as collection vial were kept covered with crushed ice mixed with salt at a temperature of −10°C, which was constantly monitored with a digital thermometer (Minipa, MVP363, China).
Storage and Preparation of the Exhaled Breath CondensateThe vials of the breath condensate obtained were kept in ice and quickly transported to a freezer, where they were maintained at −70°C for later analysis. For the analysis, the EBC of each individual was deproteinized with pure ethanol (4°C) for 30min, and right afterwards they were stored in the freezer for 30min (−20°C). After this period, they were centrifuged at 4000rpm for 10min using a refrigerated centrifuge.
Evaluation of the NOThe NO in the EBC was determined by means of chemiluminescence.4,14 Briefly, the detection of NO is based on the observation of the reaction of the ozone with NO, which produces NO2 and, when stable, can be measured by chemiluminescence, as it emits a light radiation that is proportional to the concentration of NO in the exhaled air. The luminescence generated is determined with a sensitive photomultiplier tube and is directly proportional to the NO values. The analysis is done in the Nitric Oxide Analyzer, 280i NOA (Sievers, Boulder, CO, USA).
Statistical AnalysisIn order to verify the distribution of the data, we used the Shapiro–Wilk test and Q–Q charts. The results are presented as means±standard deviation and/or medians and quartiles. For the comparisons, the Mann–Whitney U and the Kruskal–Wallis tests were used. An estimation of the curve was done using a regression procedure in order to verify the relationship between the variables. All the statistical procedures were done with the SPSS computer software, version 15.0.
ResultsTable 2 shows the demographic variables. No adverse effects came about either during or after the EBC collection procedure. The total cost of the device, including the glass tube and the unidirectional respiratory valves was $120.20 ($56.68 and $63.52, respectively).
Demographic Data.
| Age Groups, Years | |||||
| 20–30 | 31–40 | 41–50 | 51–60 | 61–70 | |
| Women/men | 21/14 | 7/11 | 18/6 | 14/6 | 14/5 |
| Mean age, years | 26.7±1.45 | 33.8±3.0 | 45.35±2.5 | 54.7±3.0 | 63.81±2.74 |
| Weight, kg | 69.84±18 | 83.55±15.3 | 68.47±13.28 | 68.12±15.33 | 68.35±11.53 |
| Height, cm | 163.70±29 | 171.88±8.83 | 164.45±9.02 | 147.61±50.69 | 160.85±7.1 |
| Tidal volume, ml | 554 (456–652)a | 594.01 (455–732)a | 552 (428–675)a | 465 (315–615)a | 528 (396–659)a |
| Minute volume, l | 8.67 (7.18–10.16)a | 8.82 (7.03–10.62)a | 8.41 (6.93–9.88)a | 9.90 (6.81–12.99)a | 9.45 (7.43–11.47)a |
| Respiratory rate, min | 12.4 (11.20–13.61)a | 12.70 (11.05–14.50)a | 12.95 (11.32–14.57)a | 13.55 (11.56–15.53)a | 15.05 (11.97–18.14)a |
The mean volume of the EBC was 1.0±0.6ml. The total volume of the EBC during collection was 95.3±42l. The mean NO in the EBC was 12.99±14.38μM (median, 8.72; C1–C3: 4.31–15.54μM). The differences in the NO content in the EBC between age groups were not significant (Table 3), nor were there any significant differences between the two sexes (Table 4).
Nitric Oxide (NO) in the Exhaled Breath Condensate (EBC) for Each Age Group.
| Age Groups, Years | P | ||||||
| 20–70 | 20–30 | 31–40 | 41–50 | 51–60 | 61–70 | ||
| No. | 112 | 34 | 18 | 24 | 17 | 19 | .301a |
| Mean | 12.99 | 12.54 | 10.47 | 16.99 | 9.71 | 14.09 | |
| Standard error | 1.35 | 3.02 | 2.25 | 3.96 | 1.32 | 1.92 | |
| Standard deviation | 14.38 | 17.64 | 9.58 | 19.42 | 5.47 | 8.40 | |
| 95% confidence interval | 10.30–15.69 | 6.38–18.70 | 5.70–15.23 | 8.79–25.19 | 6.89–12.52 | 10.04–18.14 | |
| Median | 8.72 | 6.90 | 6.49 | 9.14 | 9.40 | 12.45 | |
| C1–C3 | 1.11–93.45 | 4.03–13.51 | 3.10–17.50 | 3.72–20.18 | 6.58–11.25 | 9.65–19.62 | |
Nitric Oxide (NO) in the Exhaled Breath Condensate (EBC) and Volume of Condensate According to Sex.
| Sex | P Valuea | ||||||||
| Women | Men | ||||||||
| n | Mean | SD | Mean | n | Mean | SD | Mean | ||
| NO, μM | 73 | 12.96 | 13.42 | 9.82 | 39 | 13.05 | 16.21 | 8.30 | .847 |
| Exhaled volume | 73 | 97.26 | 45.35 | 83.64 | 39 | 91.66 | 35.16 | 87.40 | .837 |
| EBC volume, ml | 73 | 1.03 | 0.67 | 0.80 | 39 | 0.98 | 0.71 | 0.80 | .440 |
SD: standard deviation.
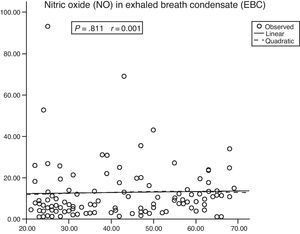
There was no correlation between the NO in the EBC and age (Fig. 2) or between the EBC volume and the NO content (P=.994; r=0.001). However, there was a significant positive correlation between the EBC volume and the volume of exhaled air (P<.001; r=0.622).
DiscussionThere is evidence that shows the important role played by NO in the physiopathology of different respiratory diseases, such as acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), asthma, pulmonary hypertension (PH), bronchiectasis, primary mucociliary dysfunction, interstitial lung disease and other diseases of the respiratory tract.5,7 Published studies have documented that asthma patients have higher levels of NO in exhaled air15,16 and levels of nitrite in EBC, compared with healthy individuals; this is possibly due to the presence of an inflammatory state.17 Monitoring the concentrations of NO in exhaled air is an emerging marker of eosinophil inflammation in asthma that makes it easier to diagnose, supervise the progression of the disease and evaluate the response to treatment.18
In patients with COPD, significantly higher levels of exhaled NO are observed when compared with smokers and health non-smokers.19 But, studies have also demonstrated that the levels of NO metabolites (NOx) in the EBC of ex-smokers with COPD are lower than in healthy individuals, non-smokers and ex-smokers.17,19
NO in the exhaled breath of humans decreases significantly after heart surgery. This may be a consequence of a reduction in the endogenous production of NO due to pulmonary vascular endothelial injury.20 This reduced production of exhaled NO may be associated with changes in respiratory mechanical function during hypoxia. Nevertheless, it has been documented that NO levels are still low after re-oxygenation, which may possibly indicate bronchial epithelial injury.21 Consequently, EBC may also be used for supervising and studying respiratory diseases and for evaluating treatment effectiveness.15,19
In the present study, we have demonstrated that the device we constructed is re-usable, effective for collecting EBC and reasonably priced. The volume of EBC that we were able to collect was sufficient to evaluate and determine NO, and we believe that it is sufficient for the analysis of other biomarkers. In addition, with this methodology we have determined the NO content in the EBC of healthy individuals and we have demonstrated that the levels of NO in the EBC of healthy individuals do not correlate with age and/or sex.
Similar to the results of the present study, Cruz et al.1 did not identify a correlation between age and NO concentration in EBC.
The NO content we detected in EBC is similar to that described by Ojoo et al.,22 Liu et al.23 and Hoffmeyer et al.,24 although greater than the values reported by Ganas et al.,25 Franklin et al.26 and Dressel et al.27 Without a doubt, the differences between devices and NO determination are responsible for this variability. In addition, it is necessary to keep in mind that the majority of this research used small-scale samples.
To date, there is not enough evidence about the correlation between the NO content in EBC and age and/or sex. As a consequence, the American Thoracic Society/European Respiratory Society (ATS/ERS)28 have recommended taking into account both age as well as sex as factors that can affect the concentration of biomarkers in EBC. For this reason, we consider that the present study makes a contribution towards answering these questions: as in other studies,1 in the present research we have not found a correlation between age or sex and the NO concentration in EBC.
Another cause for concern is the influence of ventilatory parameters on EBC and its content. The correlation found between the minute volume and the volume of EBC collected was as had been predicted, as other researchers have reported. Liu et al.29 found the same correlation and suggested that the minute volume and the tidal volume would predict the EBC volume. They also observed that sex and age did not produce a significant effect on the EBC volume. Montuschi4 also documented that the EBC volume is directly proportional to the moment at which it is obtained and the respiratory rate of exhaled air. In addition, Franklin et al.26 have demonstrated that respiratory flow does not influence the NO content in the EBC of healthy adults. This factor also deserves attention from researchers in accordance with the published abstracts from a seminar organized by the ATS.28
Another problem that merits some attention is the time of condensate collection. A collection taken over the course of 10min produced sufficient volume for the analysis of NO, and probably other markers. Longer times would translate into greater volumes, as Lema et al.30 obtained 1.5ml after 15min, and Vaughan et al.31 observed that the increase in collection time increases the volume obtained. We opted for a period of 10min because it is well tolerated and translates into a mean volume of 1–2ml, according to the recommendations in the abstracts published from the seminar organized by the ATS. Furthermore, it is necessary to verify the potential influence of temperature during collection and storage as well as to verify any influence of the material used in making the device.
More studies are needed not only to be used as a reference for the different biomarkers but also to determine whether a sequential analysis could be useful for the follow-up of the disease at hand.32
Another worrying factor is the material used in each device. The EcoScreen® and Rtube® devices are made with Teflon and aluminum,19 respectively, although manufactured devices are usually made with silicone-covered glass.13,33 We consider that borosilicate glass, although more fragile, is less expensive and also inert, which reduces the probability of influencing the marker being analyzed. Liu et al.23 compared the EcoScreen® and Rtube® devices with devices made of silicone-coated glass, but the differences were not significant when compared with non-coated glass. Soyer et al.34 also obtained greater EBC volumes using the EcoScreen® device compared with Rtube® Meanwhile, Rosias et al.35 found that the devices made with borosilicate glass, whether coated or not with silicone, were more effective for determining 8-isoprostane and albumin when compared with those made with aluminum and Teflon. They formulated the theory that glass has less adhesive properties, which means a greater number of molecules found in the EBC.
Therefore, it seems that different devices can provide different results for the same biomarker, and a consensus has not been reached regarding the most appropriate design and materials for each biomarker. Therefore, additional studies are necessary in order to define which design and material are better for a specific biomarker of interest in the EBC. Also, further research is necessary not only to evaluate the reference parameters of the different biomarkers but also to determine whether a sequential analysis may be useful for the follow-up of a certain disease.32
In conclusion, we have fabricated a reusable device that is effective for obtaining EBC and for evaluation of its NO content. In addition, we have determined the NO content in the EBC of healthy volunteers and we have demonstrated that, using this methodology, the NO content does not correlate with either age or sex.
FundingThe present research received funding from the Foundation to Support Teaching and Research (FAEPA) and the National Council for Scientific and Technological Development (CNPq) of Brazil.
Conflict of InterestsThe authors declare having no conflict of interests.
Please cite this article as: Alves Vento D, et al. Óxido nítrico en el condensado de aire espirado de individuos voluntarios sanos recolectado a través de un dispositivo reutilizable. Arch Bronconeumol. 2012;48:120–5.