Pulmonary embolism (PE) is a public health problem of the first order. Although less common than other vascular diseases, such as myocardial infarction or cerebrovascular disease, PE is equally as serious. The Spanish Medical Societies involved in the care of these patients have drawn up a consensus document that aims to update the recommendations for the diagnosis, prognosis and treatment of this disease using the best available evidence. This consensus document makes recommendations or suggestions based on the interpretation of the available evidence and its quality, risk-benefit balance of the interventions, and the cost. This was based on two documents: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed.: American College of Chest Physicians (ACCP). Evidence-Based Clinical Practice Guidelines1 and the National Institute for Health and Clinical Excellence (NICE) clinical guidelines on venous thromboembolic (VTE) diseases.2 Both documents use the GRADE system3 to establish recommendations. A systematic review of the literature published between January 2012 and March 2013 was also performed. Based on these documents and the systematic literature review, two authors (FU and DJ) prepared a manuscript that formed the basis for the final discussion (in a face-to-face meeting) by a panel composed of the coordinators of each of the participating Scientific Societies. All the recommendations or suggestions were agreed between the attendees at this session. Consensus was reached by discussion between the panel members, considering the potential risks and benefits of the interventions, routine clinical practice, recommendations of other guidelines, patient preference, and equity criteria. The authors of the document also took into account clinical situations in which a lack of evidence justifies waiting until research results become available in the future.
Concept, Pathogenesis, Risk Factors and EpidemiologyPE occurs when a detached thrombus (embolism) from any part of the venous territory becomes lodged in the pulmonary arteries. Although the origin of the embolism may be venous thrombosis in any location (upper extremities, prostatic, uterine and renal veins and right heart chambers), in most cases (90%–95%) it is a lower extremity (LE) deep vein thrombosis (DVT), often asymptomatic.
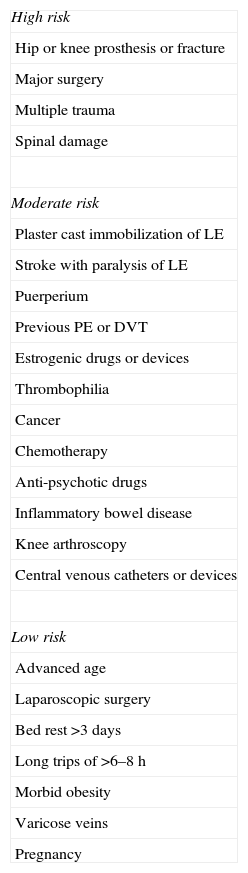
The risk factors (Table 1) for developing PE are related with one or several of the etiopathogenic mechanisms of the disease: stasis, endothelial lesion and hypercoagulability. These guidelines classify them as major or minor, depending on whether their prothrombotic risk is high or moderate-low, respectively. When the VTE is associated with precipitating risk factors, it is classified as provoked or secondary. When there are no precipitating factors, it is known as unprovoked, spontaneous or idiopathic.
Risk Factors for Venous Thromboembolism.
| High risk |
| Hip or knee prosthesis or fracture |
| Major surgery |
| Multiple trauma |
| Spinal damage |
| Moderate risk |
| Plaster cast immobilization of LE |
| Stroke with paralysis of LE |
| Puerperium |
| Previous PE or DVT |
| Estrogenic drugs or devices |
| Thrombophilia |
| Cancer |
| Chemotherapy |
| Anti-psychotic drugs |
| Inflammatory bowel disease |
| Knee arthroscopy |
| Central venous catheters or devices |
| Low risk |
| Advanced age |
| Laparoscopic surgery |
| Bed rest >3 days |
| Long trips of >6–8h |
| Morbid obesity |
| Varicose veins |
| Pregnancy |
DVT, deep vein thrombosis; LE, lower extremities; PE, pulmonary embolism.
There is an estimated incidence of PE of 1 case per 1000 population per year, although the real incidence is likely to be higher. According to data from the Spanish Ministry for Health, 22 250 cases of PE were diagnosed in 2010, with an in-hospital mortality of 8.9%.
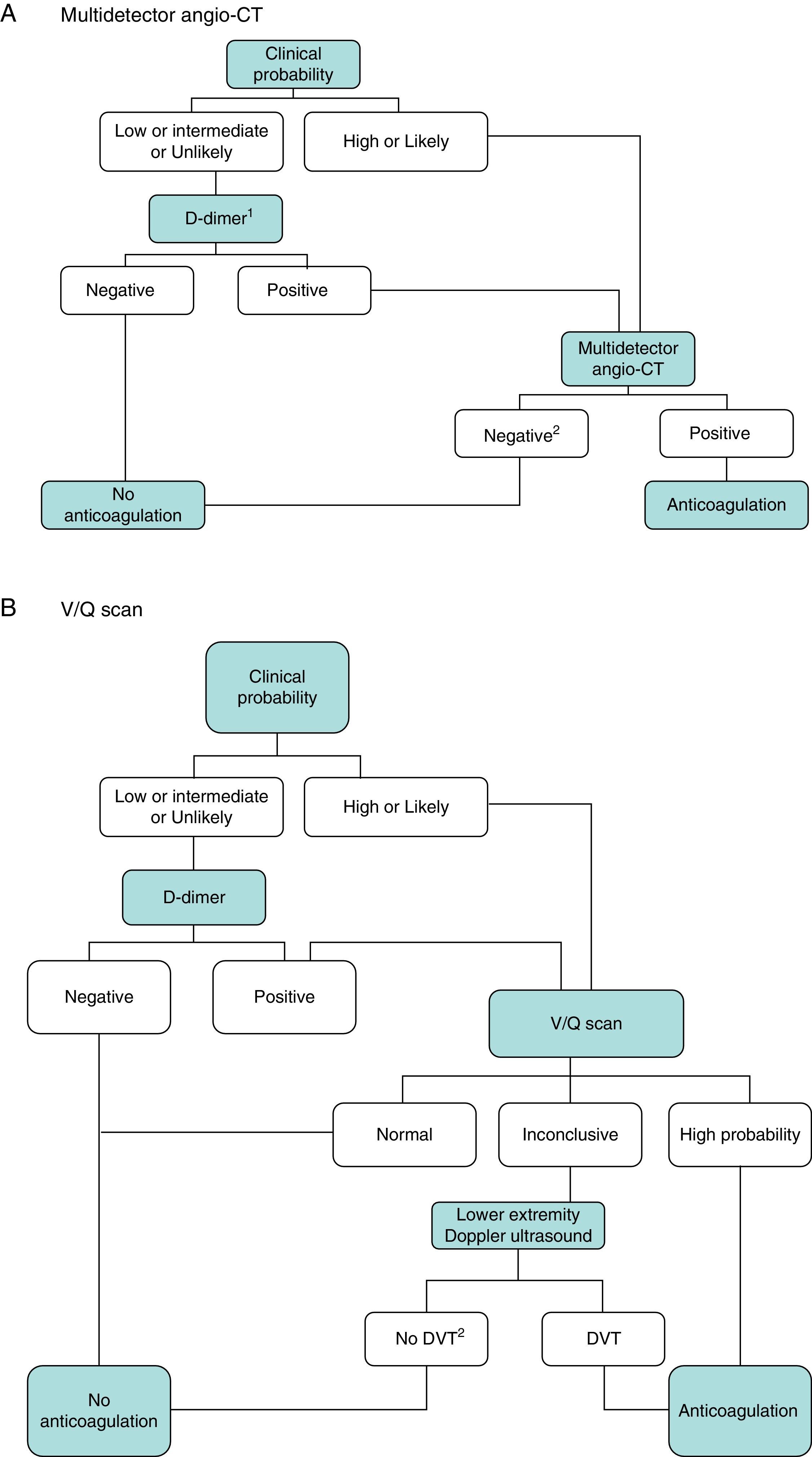
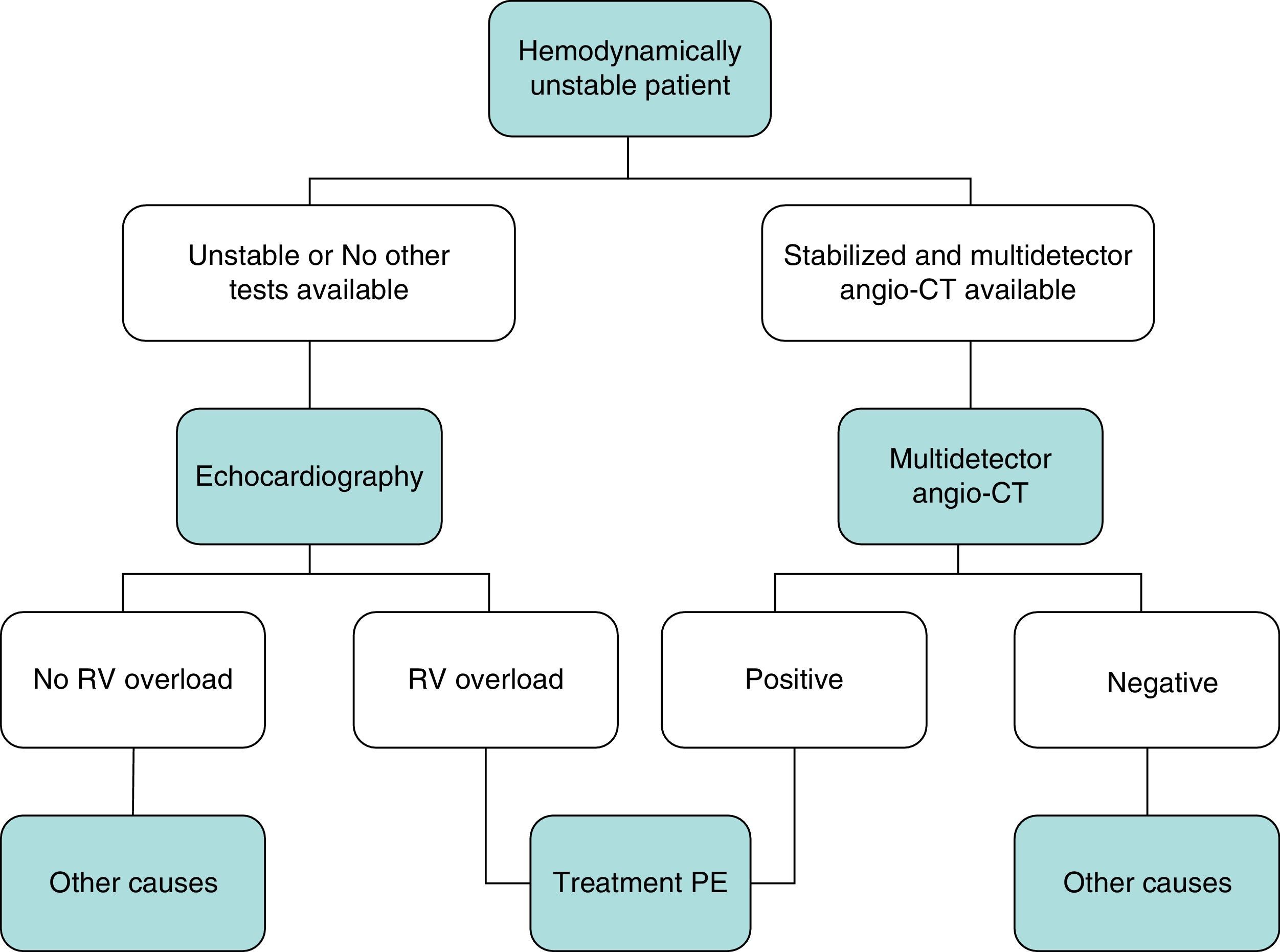
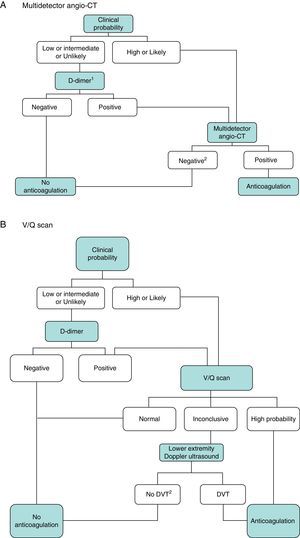
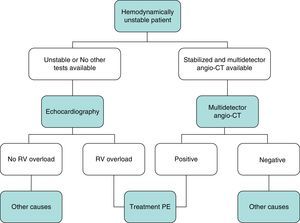
DiagnosisNo single test is sensitive and specific enough to confirm or rule out acute symptomatic PE. The diagnosis of the disease must therefore be combined with clinical suspicion, D-dimer results and imaging tests. Following widely accepted diagnostic algorithms improves the prognosis in patients assessed for suspected PE.4 A diagnostic algorithm has been proposed for hemodynamically stable patients with suspected PE (Fig. 1A and B) and another for unstable patients (Fig. 2).
Diagnostic algorithm for the hemodynamically stable outpatient. (A) Angio-CT, computed tomography angiography. 1Refers to high sensitivity D-dimer. In the case of less sensitive D-dimer, PE can only be ruled out in patients with low clinical probability or PE unlikely. 2In case of high clinical probability and negative multidetector angio-CT, additional diagnostic tests are suggested (V/Q scan and/or Doppler ultrasound of lower extremities). (B) DVT, deep vein thrombosis. 1Refers to low or intermediate probability V/Q scans. 2In case of high clinical probability, inconclusive perfusion scan, and negative ultrasound of lower extremities, the need for multidetector angio-CT should be assessed with the appropriate specialist.
The diagnostic process does not justify delays in initiating anticoagulant treatment, which should be administered early in patients with intermediate or high clinical suspicion.
- •
It is recommended to start anticoagulant treatment early (before the results of diagnostic tests become available) in patients with an intermediate or high probability of PE.
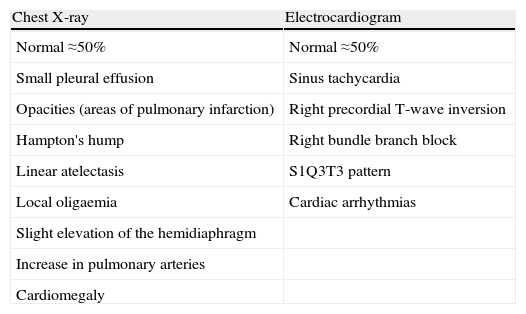
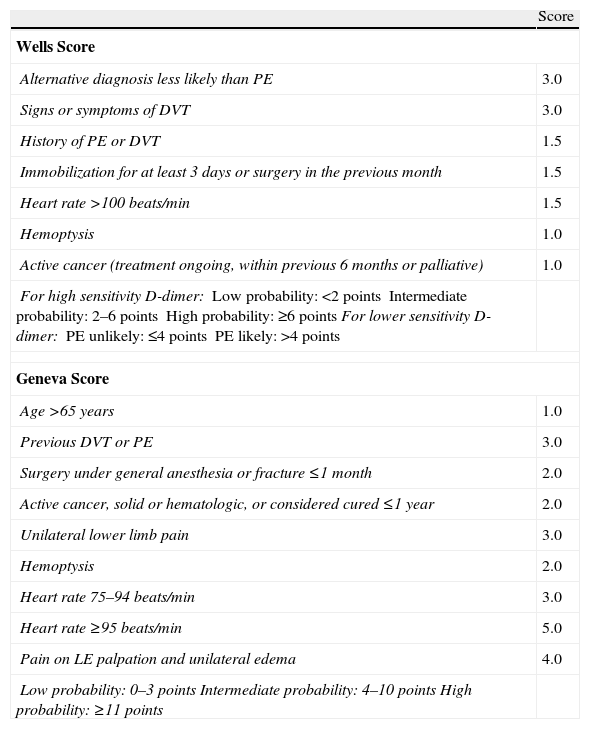
The diagnosis of acute symptomatic PE should be considered in all patients who report new onset dyspnea, worsening of their usual dyspnea, chest pain, syncope or hypotension with no alternative explanation, particularly when the basic complementary tests (chest X-ray, electrocardiogram and arterial blood gases) rule out other differential diagnoses. Table 2 lists some of the usual radiological and electrocardiographic findings in PE. Standardized testing using clinical prediction rules classifies patients into categories with different PE prevalence (approximately 10% for low probability, 25% for intermediate probability and >60% for high probability) and facilitates the interpretation of other diagnostic tests. The Wells and Geneva scores (Table 3) have been the most extensively validated.
- •
The use of adequately validated clinical scales (Wells or Geneva) is recommended as a first step in the diagnostic approach to the hemodynamically stable patient with suspected PE.
Chest X-ray and Electrocardiogram in Acute Symptomatic Pulmonary Embolism.
| Chest X-ray | Electrocardiogram |
| Normal ≈50% | Normal ≈50% |
| Small pleural effusion | Sinus tachycardia |
| Opacities (areas of pulmonary infarction) | Right precordial T-wave inversion |
| Hampton's hump | Right bundle branch block |
| Linear atelectasis | S1Q3T3 pattern |
| Local oligaemia | Cardiac arrhythmias |
| Slight elevation of the hemidiaphragm | |
| Increase in pulmonary arteries | |
| Cardiomegaly |
Scoring Scales for Grading the Clinical Likelihood of Acute Symptomatic Pulmonary Embolism.
| Score | |
| Wells Score | |
| Alternative diagnosis less likely than PE | 3.0 |
| Signs or symptoms of DVT | 3.0 |
| History of PE or DVT | 1.5 |
| Immobilization for at least 3 days or surgery in the previous month | 1.5 |
| Heart rate >100 beats/min | 1.5 |
| Hemoptysis | 1.0 |
| Active cancer (treatment ongoing, within previous 6 months or palliative) | 1.0 |
| For high sensitivity D-dimer:Low probability: <2 pointsIntermediate probability: 2–6 pointsHigh probability: ≥6 pointsFor lower sensitivity D-dimer:PE unlikely: ≤4 pointsPE likely: >4 points | |
| Geneva Score | |
| Age >65 years | 1.0 |
| Previous DVT or PE | 3.0 |
| Surgery under general anesthesia or fracture ≤1 month | 2.0 |
| Active cancer, solid or hematologic, or considered cured ≤1 year | 2.0 |
| Unilateral lower limb pain | 3.0 |
| Hemoptysis | 2.0 |
| Heart rate 75–94 beats/min | 3.0 |
| Heart rate ≥95 beats/min | 5.0 |
| Pain on LE palpation and unilateral edema | 4.0 |
| Low probability: 0–3 pointsIntermediate probability: 4–10 pointsHigh probability: ≥11 points | |
DVT, deep vein thrombosis; LE, lower extremities; PE, pulmonary embolism.
D-dimer is a fibrin degradation product present in the thrombus, generated when the clot undergoes proteolysis by plasmin. It is a highly sensitive test, but has low specificity, because the finding of high levels may also be associated with other clinical situations such as advanced age, infection, cancer, pregnancy or hospital admission.
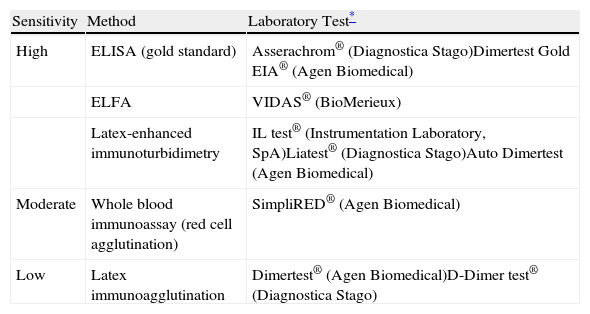
In normotensive patients with a low or intermediate probability of PE, a negative high sensitivity (≥95%) D-dimer (<500ng/ml) excludes the diagnosis of PE. In patients who do not receive anticoagulant therapy, the incidence of VTE in the following 3 months is 0.14% (95% confidence interval [CI], 0.05–0.41).5 Low or moderate sensitivity methods for determination of D-dimer (<95%) (Table 4) only exclude the disease in the group of patients with low clinical probability (or with PE unlikely according to the dichotomized Wells score).
- •
It is recommended that the sensitivity of the D-dimer method used in each setting is known.
- •
A negative high sensitivity D-dimer excludes PE in patients with low or intermediate clinical probability.
- •
A negative moderate or low sensitivity D-dimer excludes PE in patients with low clinical probability (or PE unlikely).
- •
It is recommended not to measure D-dimer in patients with a high probability of PE.
D-Dimer Measurement Techniques for Acute Symptomatic Pulmonary Thromboembolism.
| Sensitivity | Method | Laboratory Test* |
| High | ELISA (gold standard) | Asserachrom® (Diagnostica Stago)Dimertest Gold EIA® (Agen Biomedical) |
| ELFA | VIDAS® (BioMerieux) | |
| Latex-enhanced immunoturbidimetry | IL test® (Instrumentation Laboratory, SpA)Liatest® (Diagnostica Stago)Auto Dimertest (Agen Biomedical) | |
| Moderate | Whole blood immunoassay (red cell agglutination) | SimpliRED® (Agen Biomedical) |
| Low | Latex immunoagglutination | Dimertest® (Agen Biomedical)D-Dimer test® (Diagnostica Stago) |
ELFA, enzyme-linked fluorescence assay; ELISA, enzyme-linked immunosorbent assay.
The most commonly used imaging tests are multidetector chest computed tomography angiography (angio-CT), ventilation/perfusion (V/Q) lung scan and venous ultrasound of the LE, with or without Doppler (DU).
Multidetector Computed Tomography AngiographyMultidetector angio-CT is currently the imaging test of choice for the diagnosis of PE. In a systematic review and meta-analysis6 that included 2020 patients from 3 studies, the thromboembolic risk in patients who did not receive anticoagulant treatment based on a negative angio-CT was 1.2% (95% CI, 0.8–1.8), with a risk of fatal PE of 0.6%.
PIOPED II7 was a multicenter study that evaluated the diagnostic usefulness of 4, 8 or 16 detector angio-CT in patients with suspected PE. The overall sensitivity was 83% (95% CI, 76–92) and the specificity was 96% (95% CI, 93–97). In this study, the negative predictive value (NPV) of multidetector angio-CT increased marginally when venography was added in the same examination. It should be noted that the NPV of multidetector angio-CT was only 60% in patients with a high clinical probability of PE.
Multidetector angio-CT provides an effective dose of radiation of 7milliSievert, equivalent to 2 years of natural background radiation, which translates into a low additional lifetime risk of fatal cancer.8 Existing equipment enables the test to be performed with a reduced radiation dose. Nevertheless, the indication in fertile women must be justified in terms of the risk-benefit balance. In pregnant women, it is advisable to perform a perfusion scan before multidetector angio-CT, due to the lower radiation, provided that the chest X-ray is normal.9 If multidetector angio-CT is done, breast protection is recommended. It is not known if it has any effect on breast milk during breastfeeding. In patients with renal failure, angio-CT may be performed following local protocols for prevention of contrast-induced nephropathy.
- •
A negative multidetector angio-CT (technically adequate) rules out PE, except in patients with a high clinical probability of the disease.
- •
It is recommended that additional diagnostic tests be carried out in patients with suspected PE and inconclusive multidetector angio-CT.
- •
It is suggested that additional diagnostic tests be carried out in patients with a high suspicion of PE and negative multidetector angio-CT.
- •
It is recommended not to perform CT venography routinely to increase the diagnostic yield of multidetector angio-CT.
The V/Q scan has been replaced by multidetector angio-CT as the diagnostic test of choice. At present, it is generally reserved for patients with iodinated contrast allergy, some cases with renal failure or for pregnant women with suspected PE in which the DU of the LE was negative, providing that the chest x-ray was normal.
In the PIOPED I study,10 the NPV of a normal scan was 97% and the positive predictive value (PPV) of a high-probability scan was 85%–90%. However, the scan was diagnostic (normal or high probability) in only 30%–50% of patients. In the remaining patients, the V/Q scan was inconclusive (low, intermediate or indeterminate probability).
- •
A normal V/Q scan rules out clinically significant PE.
- •
A high-probability V/Q scan confirms PE in patients with an intermediate or high probability of the disease.
- •
It is recommended that additional diagnostic tests be carried out in patients with suspected PE and inconclusive V/Q scan.
DU is the method of choice for the detection of concomitant DVT in patients with PE. The main diagnostic criterion is lack of compressibility of the venous lumen. It is particularly sensitive and specific in patients with DVT symptoms and in the femoropopliteal territory, but its yield decreases when the DVT is asymptomatic or located in the sural territory. Approximately 50% of patients with acute symptomatic PE have concomitant DVT at the time of diagnosis, only half of whom are symptomatic. It is presently reserved for use in patients with discrepancy between the clinical probability and the result of the thoracic imaging tests, for patients with inconclusive thoracic tests, and for pregnant patients as a first examination in the diagnostic algorithm.
- •
DU is recommended as a first examination in pregnant patients with suspected PE.
A priori, this test does not differ from multidetector angio-CT in obtaining images of the pulmonary arterial tree. It has the advantage that it uses gadolinium (which does not contain iodine) as a contrast and does not radiate patients. In the PIOPED III study, its sensitivity was 78% and specificity was 99%.11 On extending the study with magnetic resonance venography, the sensitivity increased to 92% with a specificity of 96%. The examination was technically inadequate in 25% of patients, which is its major limitation. It may be reserved for patients with iodinated contrast allergy. In the case of severe renal failure (creatinine clearance <30ml/min), it is contraindicated due to the toxicity of gadolinium. It should be avoided in pregnancy and breastfeeding, although there is no clear evidence of teratogenic effects.
- •
It is recommended not to perform pulmonary magnetic resonance angiography routinely for the diagnosis of patients with suspected PE.
In general, transthoracic echocardiography is not useful in the diagnostic algorithm of patients with suspected PE. Although multidetector angio-CT is also the diagnostic test of choice in hemodynamically unstable patients with suspected PE, bedside echocardiography may provide very valuable diagnostic information in centers in which multidetector angio-CT is not available, or in cases in which the patient's instability prevents their transfer to the radiology department. In critically ill patients, the absence of echocardiography signs of right-sided heart dysfunction or overload rules out PE as a cause of hemodynamic compromise.
- •
It is recommended not to perform transthoracic echocardiography routinely for the diagnosis of stable patients with suspected PE.
PE is a disease with a wide spectrum of clinical manifestations, with different prognoses and treatment. The most important prognostic factor is the patient's hemodynamic status at the time of diagnosis.12High-risk PE (previously known as massive PE), which is characterized by hypotension or shock, accounts for around 5% of cases and is associated with early mortality in at least 15%. Thrombolytic treatment is usually recommended for these patients.
However, most patients with PE present with few symptoms. Early mortality in hemodynamically stable patients diagnosed with PE varies between 2% and 10%. Risk stratification of normotensive patients with PE should be used to identify a subgroup of patients with a low risk of all-cause mortality, who may benefit from early discharge or even outpatient treatment of their disease, and a subgroup of patients with a higher risk of complications associated with the PE itself (intermediate-risk PE, previously sub-massive PE), who may benefit from aggressive treatments for their disease (intensive monitoring, fibrinolysis).13
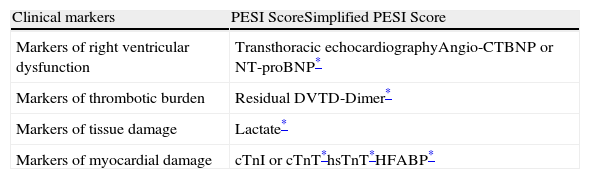
Prognostic FactorsThe most commonly used prognostic factors in normotensive patients with PE are summarized in Table 5. In general, these tools assess the patient's clinical condition and general health status, right ventricular dysfunction, the thrombotic burden and myocardial damage.
Prognostic Tools for Hemodynamically Stable Patients With Acute Symptomatic Pulmonary Embolism.
| Clinical markers | PESI ScoreSimplified PESI Score |
| Markers of right ventricular dysfunction | Transthoracic echocardiographyAngio-CTBNP or NT-proBNP* |
| Markers of thrombotic burden | Residual DVTD-Dimer* |
| Markers of tissue damage | Lactate* |
| Markers of myocardial damage | cTnI or cTnT*hsTnT*HFABP* |
Angio-CT, computed tomography angiography; BNP, brain natriuretic peptide; cTnI, cardiac troponin I; cTnT, cardiac troponin T; DVT, deep vein thrombosis; HFABP, heart fatty acid binding protein; hsTnT, high sensitivity troponin T; PESI, Pulmonary Embolism Severity Index.
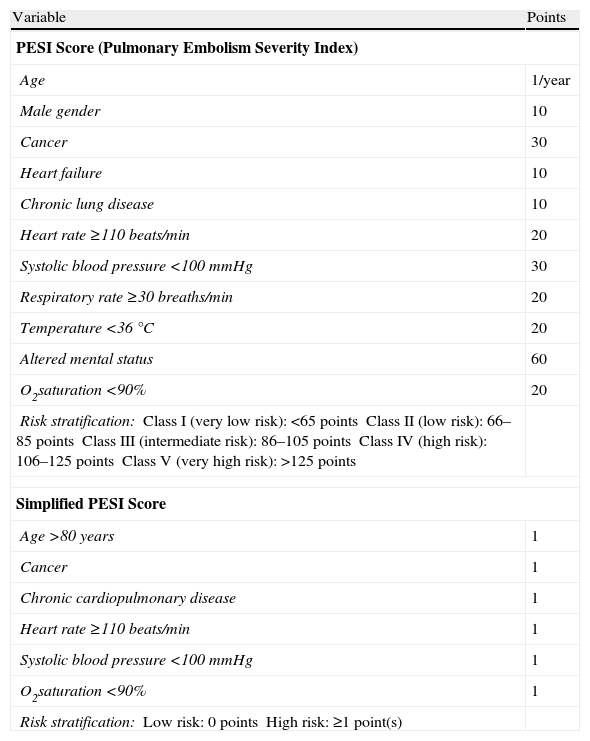
The most useful tools for classifying low risk patients with acute symptomatic PE are the prognostic clinical scores. The combination of a set of variables that takes into account the age, comorbidity and cardiovascular repercussion of PE reliably identifies a subgroup of patients (approximately 30%) with a less than 2% risk of early mortality. The Pulmonary Embolism Severity Index (PESI) and simplified PESI (sPESI) clinical scores (Table 6) have been extensively validated as excellent tools for the identification of these low risk patients.14,15 The sPESI score is easier to use than the original, while retaining its prognostic ability.
Prognostic Scores in Patients With Acute Symptomatic Pulmonary Embolism.
| Variable | Points |
| PESI Score (Pulmonary Embolism Severity Index) | |
| Age | 1/year |
| Male gender | 10 |
| Cancer | 30 |
| Heart failure | 10 |
| Chronic lung disease | 10 |
| Heart rate ≥110 beats/min | 20 |
| Systolic blood pressure <100mmHg | 30 |
| Respiratory rate ≥30 breaths/min | 20 |
| Temperature <36°C | 20 |
| Altered mental status | 60 |
| O2saturation <90% | 20 |
| Risk stratification:Class I (very low risk): <65 pointsClass II (low risk): 66–85 pointsClass III (intermediate risk): 86–105 pointsClass IV (high risk): 106–125 pointsClass V (very high risk): >125 points | |
| Simplified PESI Score | |
| Age >80 years | 1 |
| Cancer | 1 |
| Chronic cardiopulmonary disease | 1 |
| Heart rate ≥110 beats/min | 1 |
| Systolic blood pressure <100mmHg | 1 |
| O2saturation <90% | 1 |
| Risk stratification:Low risk: 0 pointsHigh risk: ≥1 point(s) | |
The value of combining the clinical scores and some biochemical markers (particularly brain natriuretic peptide [BNP] or high sensitivity troponin [hsTnT]) or imaging tests (transthoracic echocardiogram or DU) for the identification of this group of low risk patients has not been completely clarified.
- •
It is recommended to use well validated prognostic clinical scores (PESI or simplified PESI) as a first step for the identification of patients with low-risk PE.
The most widely used method in clinical practice for evaluating right ventricular function is transthoracic echocardiography. Various quantitative parameters (right ventricular end-diastolic diameter in the parasternal long axis view, ventricular end-diastolic diameter ratio in the apical 4C projection, tricuspid regurgitation peak velocity, diameter of the inferior vena cava, pulmonary artery acceleration time or TAPSE [Tricuspid Annular Plane Systolic Excursion]) have been used to estimate the degree of ventricular dysfunction. Its use for the identification of patients with intermediate-risk PE is limited by its operator-dependence, cost and lack of continuous availability in many centers. Furthermore, there is no echocardiographic pattern of right ventricular dysfunction that is sufficiently reliable as to justify, on its own, the use of fibrinolytic treatment.16
The images generated by angio-CT not only enable the diagnosis of PE to be confirmed or discarded, but they can also assess the extension of the arterial obstruction and the presence or not of right ventricular dilatation.17,18 Although ventricular size volumetric reconstruction studies may offer a more accurate prognostic assessment, more recent studies use the ratio between the ventricular diameters as a parameter of ventricular dysfunction (with different cut-off points). Its isolated use for identifying patients with intermediate-risk PE who could benefit from fibrinolytic treatment is not recommended.
Various meta-analyses have demonstrated the prognostic value of myocardial ischemia (identified by elevated troponin I or T levels) in hemodynamically stable patients with PE, although the power of the association is not sufficiently robust as to justify therapeutic escalation.19,20 Cardiomyocyte stress causes release of natriuretic peptides into the circulation (BNP and NT-pro-BNP).21,22 Several studies and meta-analyses suggest that they are useful for identifying patients with intermediate-risk PE. However, the sensitivity of these cardiac biomarkers for death due to PE is insufficient to establish the indication for thrombolytic treatment.
An association has been shown between the persistence of thrombotic material in the lower extremity deep vein system and short- and mid-term mortality of patients with PE.23 The presence of concomitant DVT, together with other imaging tests and cardiac biomarkers, may serve to identify a patient profile with a particularly high risk of complications associated with the PE itself.
- •
A combination of prognostic tests (which identify right ventricular dysfunction, myocardial ischemia, cardiomyocyte stress or thrombotic burden) is suggested for identifying patients with intermediate risk PE.
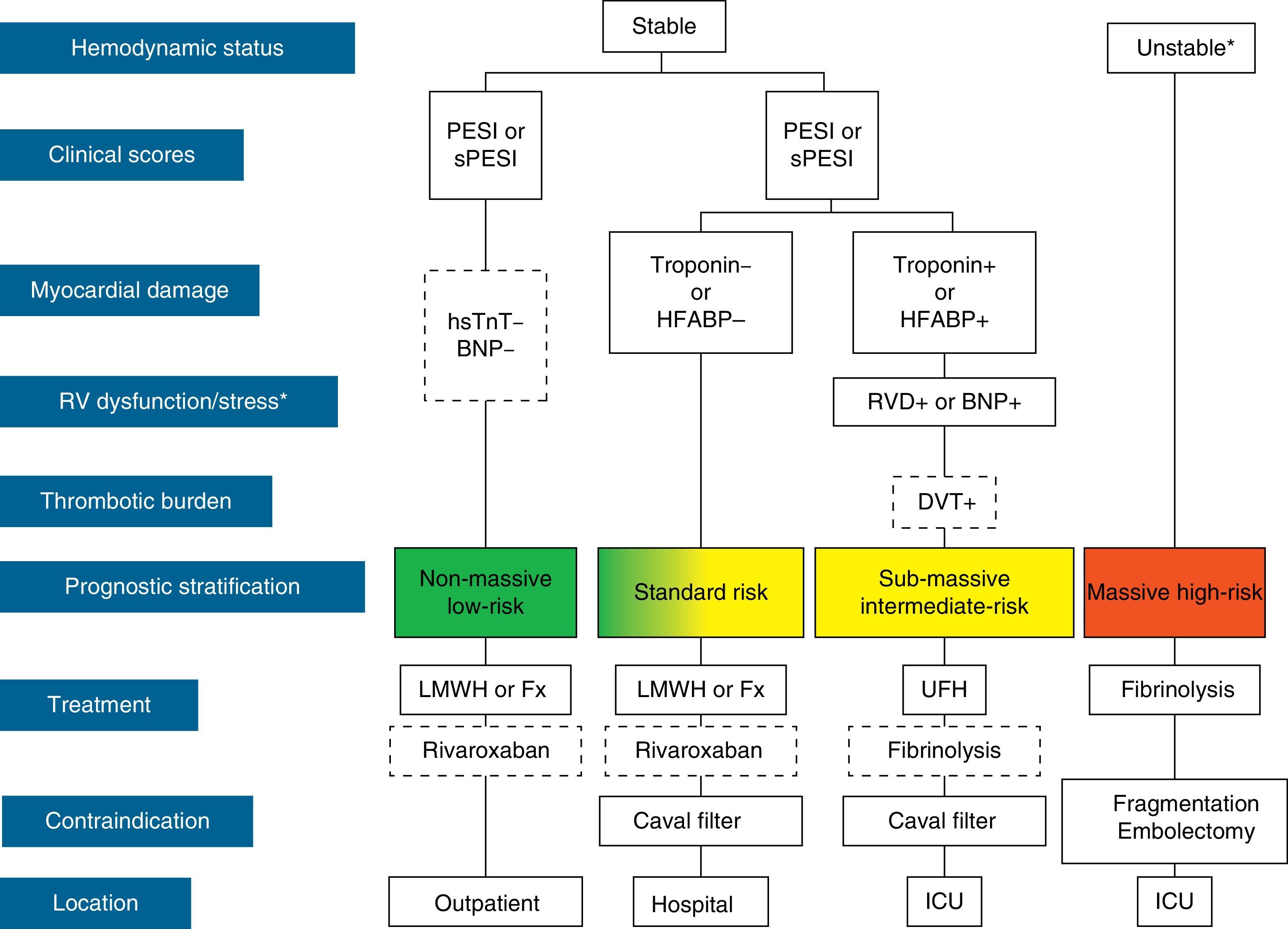
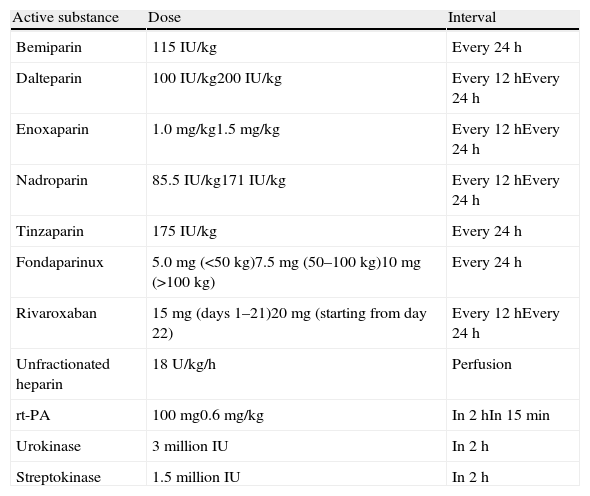
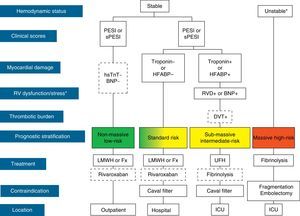
The initial treatment of PE is aimed at medical stabilization of the patient and symptom relief, resolution of the vascular obstruction and prevention of recurrence. The priority in achieving these objectives depends on the severity of the patient. On most occasions, all the objectives are reached with conventional anticoagulant treatment, which prevents progression of the clot while the endogenous fibrinolytic system resolves the vascular obstruction and collateral circulation develops. A minority of patients, usually those with hemodynamic instability (high-risk PE) or contraindication for anticoagulation, require other pharmacological treatments (thrombolytics) or mechanical measures (vena caval filters) to accelerate lysis of the clot or prevent its embolization to the lungs1 (Fig. 3). Table 7 lists the administration guidelines for drugs approved for treatment of the acute phase of PE.
Prognostic stratification and treatment of acute pulmonary embolism. *Cardiogenic shock or sustained systolic blood pressure <90mmHg, not due to hypovolemia, sepsis or cardiac arrhythmias. The broken line indicates absence of definitive evidence (fibrinolysis) or extensive clinical experience (rivaroxaban). For situations not considered in the algorithm, hospitalization and conventional anticoagulant therapy is recommended. BNP, brain natriuretic peptide; DVT, deep vein thrombosis; Fx, fondaparinux; HFABP, heart fatty acid binding protein; hsTnT, high sensitivity troponin T; ICU, intensive care unit; LMWH, low molecular weight heparin; PE, pulmonary embolism; PESI, Pulmonary Embolism Severity Index; RVD, right ventricular dysfunction; sPESI, simplified PESI; UFH, unfractionated heparin.
Treatment Guidelines for the Acute Phase of Pulmonary Embolism.*
| Active substance | Dose | Interval |
| Bemiparin | 115IU/kg | Every 24h |
| Dalteparin | 100IU/kg200IU/kg | Every 12hEvery 24h |
| Enoxaparin | 1.0mg/kg1.5mg/kg | Every 12hEvery 24h |
| Nadroparin | 85.5IU/kg171IU/kg | Every 12hEvery 24h |
| Tinzaparin | 175IU/kg | Every 24h |
| Fondaparinux | 5.0mg (<50kg)7.5mg (50–100kg)10mg (>100kg) | Every 24h |
| Rivaroxaban | 15mg (days 1–21)20mg (starting from day 22) | Every 12hEvery 24h |
| Unfractionated heparin | 18U/kg/h | Perfusion |
| rt-PA | 100mg0.6mg/kg | In 2hIn 15min |
| Urokinase | 3 million IU | In 2h |
| Streptokinase | 1.5 million IU | In 2h |
IU, international units; rt-P, recombinant tissue plasminogen activator.
For decades, unfractionated heparin (UFH) has been the drug of choice for the treatment of PE. It exerts its anticoagulant action by binding to antithrombin and potentiating its effect in the inactivation of a series of activated coagulation factors, mainly thrombin (IIa). It is usually administered intravenously (iv) as a continuous infusion, but it is also safe and effective using the subcutaneous route (sc). It requires monitoring using the activated partial thromboplastin time (APTT), which should be 1.5–2.5 times the control value, so an initial infusion rate of 18U/kg/h is used. As well as continuous infusion, an 80U/kg bolus is usually administered to achieve a more rapid anticoagulant effect. Patients who are treated with lower doses and who do not reach a therapeutic APTT in the first few days of treatment have an increased risk of recurrent VTE. It is not known if there is a relationship between sub-therapeutic APTT levels and recurrence in patients who are treated initially with the recommended doses. UFH is currently reserved for patients in whom the use of fibrinolytic treatment is considered (intermediate- or high-risk PE), and for patients at a high risk of bleeding who are to receive anticoagulant therapy. For patients with severe renal failure (creatinine clearance <30ml/min), some indirect evidence suggests that low molecular weight heparins (LMWH) (at the doses recommended in the Summary of Product Characteristics) could be safer and more effective than UFH.24
LMWH are prepared from the fractionation of UFH by chemical or enzymatic methods. They have potent antiXa action (greater than that of UFH). Most studies suggest that LMWH and UFH are equivalent in terms of efficacy (recurrent VTE) and safety (major bleeding) for treatment of the acute phase of VTE. In a meta-analysis that included 1951 patients from 12 studies comparing LMWH with UFH, LMWH were associated with fewer recurrent VTE (odds ratio [OR] 0.63; 95% CI, 0.33–1.18) and fewer major bleeding events (OR 0.67; 95% CI, 0.36–1.27), with no differences in mortality (OR 1.20; 95% CI, 0.59–2.45).25
Fondaparinux is a synthetic pentasaccharide that selectively inhibits factor Xa without inactivating thrombin. It is administered subcutaneously once daily at weight-adjusted doses and does not require monitoring. The Matisse investigators evaluated its efficacy and safety in the treatment of acute DVT and PE.26 Compared with UFH, they did not observe any differences in the rate of recurrent thromboembolic events (1.3% vs 1.7% in the acute phase), major bleeds (1.3% vs 1.1%) or mortality in the first 3 months of follow-up. A potential advantage of this drug is that it is not associated with heparin-induced thrombocytopenia (HIT).
- •
The use of LMWH or fondaparinux instead of UFH is suggested in hemodynamically stable patients with acute PE.
- •
The use of LMWH at doses adjusted to the UFH is suggested in patients with acute PE and severe renal failure.
Studies demonstrating that heparin treatment for 5 days instead of 10 is a safe, effective practice also showed that oral anticoagulants could be started on the first day of anticoagulation, without losing efficacy. Two clinical trials with warfarin have shown that: (1) loading doses (which may cause hemorrhages) should be avoided without this incurring a delay in reaching therapeutic INR levels; (2) commencing vitamin K antagonists (VKA) at lower doses avoids excessive falls in protein C levels, which would theoretically induce a state of hypercoagulability.
- •
In hemodynamically stable patients with PE, it is recommended that parenteral anticoagulation be maintained for at least 5 days, and until the INR is >2.0 for 24h.
New oral anticoagulants have recently been introduced that act differently to the VKA, among which are dabigatran (direct thrombin inhibitor), rivaroxaban and apixaban (factor Xa inhibitors). Rivaroxaban is a direct selective factor Xa inhibitor. In the joint analysis of two clinical trials (for DVT and PE), rivaroxaban was associated with an efficacy similar to that of standard treatment (hazard ratio [HR] 0.87; 95% CI, 0.66–1.19) while major bleeding was reduced to half (HR 0.54, 95% CI, 0.37–0.79).27,28 Its oral administration, at a dose of 15mg twice daily for 3 weeks followed by 20mg once daily, could provide a simple, single-drug approach to the acute and long-term treatment of PE.
Apixaban has been evaluated in a clinical trial for the treatment of patients with DVT or PE (34%) for the first 6 months after a thrombotic event.29 Compared with standard treatment, apixaban showed similar efficacy (relative risk [RR] 0.84; 95% CI, 0.60–1.18) and a statistically significant reduction in major bleeding (RR 0.31; IC 95%, 0.17–0.55) and clinically relevant non-major bleeding (RR 0.48; 95% CI, 0.38–0.60). It was administered orally, at a dose of 10mg twice daily for the first 7 days followed by 5mg twice daily.
The efficacy and safety of dabigatran has not been evaluated during the first 10 days (on average) of treatment of acute symptomatic PE.
- •
Treatment with rivaroxaban as monotherapy is suggested in hemodynamically stable patients with PE.
The results of a clinical trial and some cohort studies suggest that, compared with hospitalization, outpatient treatment in low risk patients is equally effective and safe in terms of recurrent VTE, bleeding events and mortality.30 Outpatient treatment can be considered in patients with PE who meet the following requirements: (1) clinically stable with good cardiopulmonary reserve, and a low risk validated clinical score (e.g. PESI or sPESI), (2) good social support with rapid access to medical care and (3) expected treatment compliance.
- •
In patients with low-risk PE and adequate home conditions, early discharge is suggested instead of standard discharge (more than 5 days admission).
Several meta-analyses have shown that early mobilization is a safe practice in patients with proximal DVT.31 The evidence is less consistent for patients with symptomatic PE. The presence of concomitant DVT worsens the prognosis in patients with PE, particularly when associated with right ventricular dysfunction and myocardial ischemia.32
- •
Early mobilization is suggested in patients with low-risk PE.
- •
Rest is suggested for the first few days of treatment in patients with intermediate-risk PE.
Thrombolytic treatment accelerates clot lysis and hemodynamic improvement occurs more rapidly than with UFH treatment, although there are no differences in the residual thrombosis after 5–7 days. Analysis of a subgroup of patients in the Urokinase Pulmonary Embolism Trial (UPET), which compared urokinase followed by UFH or UFH alone, showed that fibrinolytic treatment reduces mortality in patients with shock secondary to massive PE.33 Based on these findings, and in the absence of a high risk of bleeding, thrombolytic treatment is indicated in patients with acute symptomatic PE and hemodynamic instability (defined as cardiogenic shock or sustained systolic blood pressure <90mmHg, not due to hypovolemia, sepsis or cardiac arrhythmias).34 Hemodynamic instability is rare, but up to half of patients with PE without hemodynamic instability have clinical and echocardiographic signs of right ventricular dysfunction (intermediate-risk PE). Although not routinely recommended,35 and pending publication of the results of the Pulmonary Embolism Thrombolysis Study (PEITHO),36 the decision to use thrombolytic treatment in a very selected group of patients will depend on their risk of bleeding and the severity of the clinical symptoms.
Thrombolytic treatment administered for 2h is safer and more effective than 12–24-h regimens. Streptokinase and recombinant tissue plasminogen activator (rt-PA) are equally effective with this short administration regimen. It should be administered via a peripheral route. Administration through a central line is not more effective and increases the risk of bleeding at the venous access insertion site.
- •
The administration of systemic fibrinolytic treatment is recommended in patients with PE and cardiogenic shock.
- •
The administration of systemic fibrinolytic treatment is suggested in patients with PE and hypotension without a high risk of bleeding.
- •
It is recommended not to administer fibrinolytic treatment in most hemodynamically stable patients with PE.
- •
In hemodynamically stable patients with intermediate-risk PE and a low risk of bleeding, particularly in the under 75s, it is suggested that the administration of fibrinolytic treatment be assessed.
Vena caval filters are indicated in PE patients with a contraindication for anticoagulation therapy. Decousus et al. published a clinical trial evaluating the usefulness of vena caval filters, as a complement to conventional anticoagulation, in patients with DVT and high-risk PE.37 The filters reduced the frequency of PE during the first 12 days of treatment, and a tendency toward a lower number of fatal episodes was detected. After 2 years, there were no differences between the two groups in terms of mortality or recurrent VTE, due to an increase in the frequency of DVT in the group treated with filters. These findings indirectly support the use of vena caval filters in patients in whom anticoagulation is contraindicated in the acute phase. A recoverable filter should be inserted when possible, and should be removed as soon as anticoagulation can be commenced.
- •
Placement of an inferior vena caval filter is recommended in hemodynamically stable patients with PE and a contraindication for anticoagulation.
In experienced centers, mechanical thrombus fragmentation is performed in patients with high-risk PE and a contraindication for the use of fibrinolytics. Pulmonary embolectomy is another method of treatment for high-risk PE. It is indicated in cases of right-sided heart thrombi, high risk of paradoxical arterial embolism or in patients with high-risk PE in whom fibrinolysis has not been effective.38 The results improve if patients undergo the procedure before developing cardiogenic shock.
- •
In patients with high-risk PE and who (i) have a contraindication for fibrinolysis, (ii) fibrinolysis has failed, or (iii) the shock is likely to cause death of the patient before the fibrinolysis is effective, the use of interventionist catheterization techniques or pulmonary embolectomy is suggested if the necessary resources and expertise are available.
Patients with acute symptomatic PE should receive supplementary oxygen to obtain saturations higher than 92%. Oxygen therapy, especially in patients with right-sided heart overload, acts as a vasodilator and may contribute to the decrease in pressure in the pulmonary arteries.
Pleuritic chest pain is a common symptom in patients with PE. It can be relieved on most occasions by administering non-steroidal anti-inflammatory agents within 24–48h. The administration of these drugs does not increase the risk of bleeding in acute PE.
The administration of fluids (<500ml) may be beneficial for increasing the cardiac output in patients with PE, low output and sustained systemic pressures. Dopamine or dobutamine can be used in patients with low output and sustained systemic pressures. For hypotensive patients with PE, adrenaline combines the beneficial effects of noradrenaline and dobutamine.
Treatment of Isolated Subsegmental Pulmonary EmbolismA meta-analysis of studies that performed chest angio-CT for suspected PE found that the incidence of subsegmental PE was 4.7% (95% CI, 2.5–7.6) and 9.4% (95% CI, 5.5–14.2) in patients undergoing single- and multidetector CT. respectively.39 There were no differences between the two groups in the incidence of thrombotic events during the first 3 months of follow-up when the patients did not receive anticoagulant therapy based on a negative angio-CT.
Treatment of Incidental Pulmonary EmbolismIncidental PE (unsuspected) is detected in approximately 2% of patients (most with cancer) in whom a chest CT is performed for reasons other than suspected PE. Some indirect evidence suggests that incidental PE worsens the prognosis in these patients.40 The recommendation for indicating anticoagulant therapy is more consistent when the incidental PE is associated with concomitant PE, the PE is lobar or in the main arteries and the risk of bleeding is not high.
- •
Anticoagulant therapy is suggested in patients with incidental PE in a segmental, lobar or main location.
The presence of right-sided heart thrombi, particularly when they are mobile, significantly worsens the prognosis in patients with PE. Some indirect evidence suggests that fibrinolysis or surgical embolectomy is more effective than conventional anticoagulant therapy in these patients.
Long-term Treatment of Pulmonary Thromboembolism (After the First 3–6 Months)Duration of TreatmentThe appropriate duration of anticoagulant treatment requires a balance between the risk of recurrent VTE and the risk of bleeding complications.
Risk of RecurrenceThe risk of recurrent VTE depends on (1) the efficacy of treatment of the acute episode, (2) a minimum duration of long-term treatment, (3) the possibility of the patient having an intrinsic risk factor for suffering a new VTE episode.
In a meta-analysis that included 2925 patients who had suffered a first episode of VTE not secondary to cancer, and who had received treatment of different durations, the risk of recurrence increased significantly for treatment durations less than 3 months (HR 1.52; 95% CI, 1.14–2.02).41 There were no differences in the risk of recurrence for treatment durations of 3 months compared to durations of 6 or more months (HR 1.19; 95% CI, 0.86–1.85). Based on this evidence, it is agreed that long-term treatment of patients with PE should have a minimum duration of 3 months.
In the aforementioned meta-analysis,41 the risk of recurrence was significantly lower for events caused by a temporary risk factor than for unprovoked events (HR 0.55; 95% CI, 0.41–0.74). In a systematic review42 that included 15 clinical trials, the risk of recurrent VTE during follow-up for patients with unprovoked VTE was 2.3–2.5 times higher than that of patients with provoked VTE, 7.9–10.6 times higher than patients with VTE provoked by a surgical risk factor, and 1.4–1.8 times higher than that of patients with VTE provoked by a non-surgical risk factor.
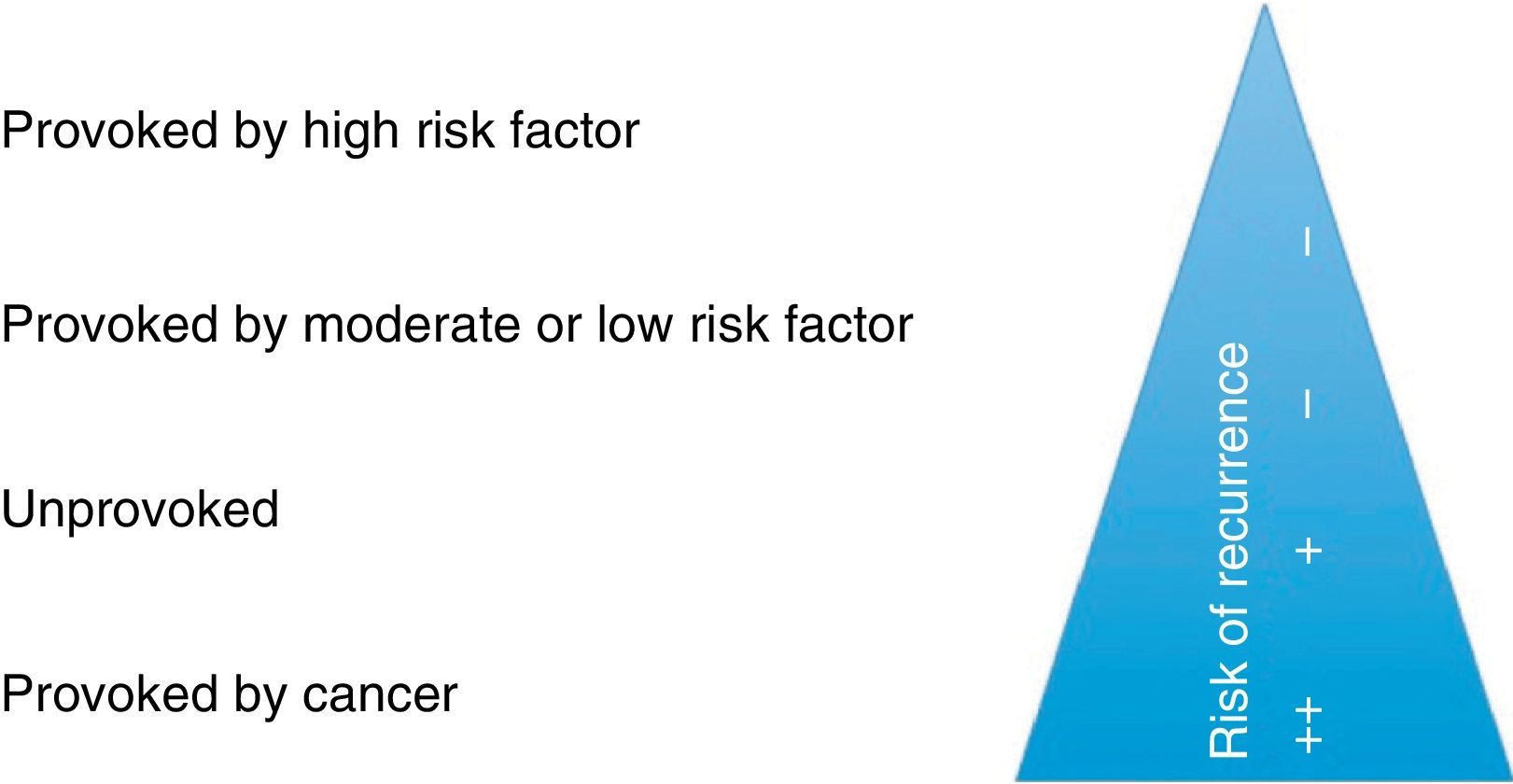
Although the literature is not consistent, for the purpose of decision-making on treatment duration, these guidelines stratify PE as provoked by a major risk factor, provoked by a minor risk factor, unprovoked or secondary to cancer (Fig. 4).
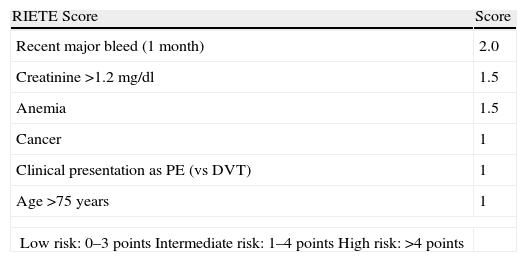
Bleeding RiskThere are no bleeding risk scales that have been sufficiently validated in patients on anticoagulant therapy for an episode of VTE. The RIETE registry43 derived a score for predicting the risk of bleeding in the first 3 months of anticoagulant therapy from 19274 patients diagnosed with DVT or PE (Table 8). Based on the weight of each of the variables on the scale, patients were classified into three risk groups: low, intermediate and high. The incidence of major bleeding in the internal validation cohort was 0.1%, 2.8% and 6.2%, respectively.
RIETE Score for Bleeding Risk in Patients With Venous Thromboembolism During the First 3 Months of Anticoagulant Treatment.
| RIETE Score | Score |
| Recent major bleed (1 month) | 2.0 |
| Creatinine >1.2mg/dl | 1.5 |
| Anemia | 1.5 |
| Cancer | 1 |
| Clinical presentation as PE (vs DVT) | 1 |
| Age >75 years | 1 |
| Low risk: 0–3 pointsIntermediate risk: 1–4 pointsHigh risk: >4 points | |
DVT, deep vein thrombosis; PE, pulmonary embolism; RIETE, Computerized Registry of Patients with Venous Thromboembolism.
After the third month of anticoagulant therapy, the evidence is more limited. The 9th edition of the ACCP1 proposes a scale for risk of bleeding from a series of variables that have been associated with bleeding events in the literature (Table 9). According to this model, the risk of a major bleed is low (0.3%) in the absence of any risk factor, moderate (0.6%) in the presence of one risk factor, and high (≥2.5%) if there are two or more risk factors. This model has not been validated in an external cohort of patients with VTE.
ACCP Score for Bleeding Risk in Patients on Anticoagulant Therapy for More Than 3 Months Due to Venous Thromboembolic Disease.*
| Variable |
| Age >65 years |
| Age >75 years |
| Previous bleed |
| Cancer |
| Cancer with metastasis |
| Renal failure |
| Liver failure |
| Thrombocytopenia |
| Previous stroke |
| Diabetes |
| Anemia |
| Anti-platelet therapy |
| Poorly controlled anticoagulation |
| Comorbidity and reduced functional capacity |
| Recent surgery |
| Frequent falls |
| Alcohol abuse |
| Low risk: 0 risk factorsModerate risk: 1 risk factorHigh risk: ≥2 risk factors |
ACCP, American College of Chest Physicians.
Two decision strategies have been developed on the duration of long-term anticoagulation in patients with PE: a population strategy and an individualized strategy. The first of these only considers provoked PE, unprovoked PE or PE secondary to cancer for making set recommendations on the duration of anticoagulant treatment. The second takes into account the clinical characteristics of each patient (age, sex, comorbidity, presentation of the event) and some other additional factor (D-dimer) to suggest individualized plans for duration of anticoagulant treatment. The authors of these guidelines suggest a mixed approach for decision-making.
- •
In patients with PE provoked by surgical transient risk factors, 3 months of anticoagulant treatment is recommended.
- •
In patients with PE provoked by non-surgical transient risk factors, 3 months anticoagulant treatment is suggested.
- •
In patients with unprovoked PE, a minimum of 3–6 months anticoagulant treatment is recommended, and it is suggested that indefinite treatment be assessed according to the balance between the risk of recurrence and the risk of bleeding.
- •
In patients with cancer, at least 3–6 months anticoagulant treatment is recommended, and it is suggested that the treatment be prolonged while the cancer is active.
- •
Indefinite anticoagulant treatment is recommended in patients with a second episode of unprovoked PE.
- •
In patients with an indication for indefinite anticoagulation, periodical reevaluation of this indication is recommended.
Event presentation. In a meta-analysis that included 2554 patients with PE or DVT who were followed-up for 5 years, the initial presentation of the thrombotic event as PE tripled the risk that the recurrent VTE was again a PE (vs DVT) (HR 3.1, 95% CI, 1.9–5.1).44 These results highlight the importance of presentation of the event, since the mortality of PE is significantly higher than that of DVT.
D-dimer. In the PROLONG45 trial, D-dimer was measured 1 month after discontinuing anticoagulant therapy in patients with a first episode of unprovoked VTE who had received at least 3 months VKA treatment. Patients with negative D-dimer did not receive anticoagulant therapy, while those with positive D-dimer were randomized to restart or discontinue anticoagulant therapy definitively. The rate of recurrent VTE during follow-up was 15% in the group with positive D-dimer who stopped anticoagulant treatment, 6.2% in the group with negative D-dimer, and 2.6% in the group with positive D-dimer who received anticoagulant treatment. These results have been confirmed in subsequent systematic reviews and meta-analyses.
Residual deep vein thrombosis. Residual DVT is defined as persistence of the organized thrombus adhered to the vein wall over time. In a systematic review and meta-analysis of 9 cohort studies and 5 randomized trials,46 it was concluded that residual DVT was modestly associated with an increase in the risk of recurrence for all patients with DVT (both provoked and unprovoked) (OR 1.5; 95%, CI 1.1–2.0), but the effect disappeared when only the subgroup of patients with unprovoked DVT was considered (OR 1.2; 95% CI, 0.9–1.7). Its limitation is that the evaluation is operator-dependent and interobserver variability is high.
First vs second episode of PE. After a second episode of VTE, the risk of recurrence is approximately 1.5 times higher than after the first episode.47 The risk of recurrence is particularly high when the second episode occurs shortly after discontinuing anticoagulant therapy.
Hereditary and acquired thrombophilia. In a meta-analysis, the risk of recurrent VTE was 1.6 (95% CI, 1.1–2.1) for patients heterozygous for factor V Leiden (1.2; 95% CI, 0.6–2.2, for the subgroup of patients with unprovoked VTE), 2.6 (95% CI, 1.2–6.0) for homozygous patients, and 1.4 (95% CI, 1.0–2.2) for patients heterozygous for the prothrombin G20210A gene.48 Given that the association between hereditary thrombophilia and the risk of recurrence is weak (if it exists), its measurement to determine the duration of anticoagulant treatment is not recommended.
Studies that have evaluated the association between the presence of antiphospholipid antibodies (APA) and the risk of recurrent VTE do not provide consistent data for deciding the duration of anticoagulation if they are detected.
Other risk factors of recurrence. Advanced age, male gender, obesity, post-thrombotic syndrome secondary to DVT concomitant with PE, some anti-psychotic drugs and certain chronic diseases such as inflammatory bowel disease have been associated with an increased risk of recurrence when anticoagulant treatment is discontinued.
Predictive Models of Recurrence for Patients With Unprovoked Pulmonary EmbolismVarious predictive models have been developed that could identify patients with unprovoked PE and low risk of recurrence. However, none have been prospectively validated. The most widely used models are the DASH (D-Dimer, Age, Sex, Hormonal treatment) score,49 Vienna nomogram,50 developed with 3 variables associated independently with the risk of recurrence: female sex, proximal DVT vs PE and D-dimer value determined after discontinuation of anticoagulant treatment, and the Canadian model,51 valid for women, which includes the following variables: age, body mass index, D-dimer determined after discontinuing anticoagulant treatment and presence of post-thrombotic symptoms or signs in the LE.
Drugs For Long-Term TreatmentThese guidelines only make recommendations or suggestions for drugs approved in Spain for this indication.
Vitamin K AntagonistsAcenocumarol and warfarin are the two dicumarol derivatives available in Spain. They interfere competitively in the metabolism of vitamin K and inhibit the production of coagulation proteins dependent on this vitamin (factors II, VII, IX and X and proteins C, S and Z). Acenocumarol has a shorter half-life and faster metabolic clearance than warfarin. Control of the therapeutic action requires monitoring expressed as the international normalized ratio (INR). An INR range between 2.0 and 3.0 has the best balance between efficacy and safety,52 and reduces the risk of recurrence by 80%. After having completed a minimum of 6 months with a range between 2.0 and 3.0, a clinical trial found that the administration of VKA with a lower range, between 1.5 and 1.9, reduced the risk of recurrence by 64% (HR 0.36; 95% CI, 0.19–0.67) without increasing the risk of major bleeding (HR 2.53; 95% CI, 0.49–13.03).53 In another clinical trial, when this regimen (range between 1.5 and 1.9) was compared with the conventional regimen (range between 2.0 and 3.0), the risk of recurrence was significantly higher (HR 2.8; 95% CI, 1.1–7.0), with no differences in the risk of major bleeding (HR 1.2; 95% CI, 0.4–3.0).52
In patients with difficulty in regular monitoring or maintaining a stable INR with VKA (more than 50% of measurements within range over a 6-month period), the balance between efficacy and safety of VKA is not guaranteed.
Low Molecular Weight HeparinsThese are indicated for at least the first 3 months of treatment of VTE in patients with active cancer, where they have been shown to be more effective than VKAs. However, as the doses for long-term treatment are not well established and their administration is parenteral, their use is not recommended as first choice in patients with VTE not secondary to cancer, although they could be an alternative for patients with difficulty in properly controlling VKA levels or unstable INR.
New Oral AnticoagulantsAt the time of writing of this consensus, the European Medicines Agency (EMA) has only approved rivaroxaban for the secondary prevention of VTE after a first episode of DVT or PE. Other anticoagulants, such as dabigatran or apixaban, already marketed for other indications, are pending approval.
The Einstein-Extension study27 randomly assigned 1197 patients who had completed 6–12 months of anticoagulation (with VKA or rivaroxaban) to receive rivaroxaban at doses of 20mg/day or placebo. During the study period, rivaroxaban significantly reduced the risk of recurrent VTE (HR 0.18; 95% CI, 0.09–0.39). There were no significant differences in the incidence of major bleeding, but rivaroxaban increased the risk of major or clinically relevant non-major bleeding (HR 5.19; IC 95%, 2.3–11.7).
In the AMPLIFY-EXT trial,54 two doses of apixaben (2.5 and 5mg, twice daily) were compared with placebo in patients with VTE who had completed 6–12 months of anticoagulant treatment (with VKA or apixaban). During the study period, apixaban significantly reduced the risk of recurrent VTE (relative risk [RR] 0.19; 95% CI, 0.11–0.33 for the 2.5mg dose; RR 0.20; 95% CI, 0.11–0.34 for the 5mg dose), with no differences in the incidence of major or clinically relevant non-major bleeding (RR 1.20; 95% CI, 0.69–2.10 for the 2.5mg dose; RR 1.62; 95% CI, 0.96–2.73 for the 5mg dose).
In two clinical trials, RE-MEDY and RE-SONATE,55 dabigatran at a dose of 150mg twice daily was compared with VKA and placebo (respectively) in patients with VTE who had completed at least 3 months of anticoagulation (with VKA or dabigatran). During the study period, dabigatran was equally as effective as the VKAs (HR 1.44; 95% CI, 0.78–2.64) and more effective than placebo (HR 0.08; 95% CI, 0.02–0.25) in reducing recurrent VTE. Dabigatran reduced the total incidence of major or clinically relevant non-major bleeding when compared with VKA (HR 0.54; 95% CI, 0.41–0.71), but these increased when compared with placebo (HR 2.92; 95% CI, 1.52–5.60).
AspirinTwo clinical trials were published in 2012, WARFASA56 and ASPIRE,57 which evaluated the efficacy and safety of aspirin compared to placebo in the secondary prevention of VTE in patients with a first unprovoked episode of PE or DVT. In the WARFASA study, aspirin was compared to placebo in patients with VTE who had completed a 6–18-month period of treatment with VKA (DVT 63%; PE 37%). During the 2 years of follow-up, aspirin significantly reduced the risk of recurrent VTE (HR 0.55; 95% CI, 0.33–0.92), without increasing bleeding episodes (HR 0.98; 95% CI, 0.24–3.96). In the ASPIRE study, aspirin was compared to placebo in patients with VTE who had completed a 6-week to 24-month period of treatment with VKA. No differences were observed in the rate of recurrent VTE (HR 0.74; 95% CI, 0.52–1.09), although there was an overall clinical benefit in the reduction of the composite of cardiovascular episodes, bleeding, and death from any cause (HR 0.67; 95% CI, 0.49–0.91).
- •
The use of VKA is recommended for most patients with PE, with a target INR of 2.5 (range 2.0–3.0), for long-term anticoagulant treatment.
- •
It is suggested not to use VKA with a target INR of 1.5–1.9 for long-term anticoagulant treatment.
- •
The use of LMWH is recommended for patients with PE secondary to cancer throughout treatment.
- •
The use of rivaroxaban for long-term anticoagulant treatment is suggested in patients with difficulty in regular monitoring or maintaining a stable INR with VKA.
- •
In patients with unprovoked PE in whom oral anticoagulation has been discontinued, it is suggested to assess the use of aspirin, at a dose of 100mg/day, once the minimum treatment duration (3 months) has been completed.
In a review of 4 studies in which serial imaging tests were carried out, the percentage of patients with residual PE was 87% at 8 days, 68% at 6 weeks, 65% at 3 months, 57% at 6 months and 52% at 11 months.58 However, in a study of 673 patients from a single center who were followed-up for 3 months, the rate of symptomatic recurrent VTE was only 3%.59 Therefore, there is disagreement between the percentage of patients with residual thrombosis and the percentage of patients with recurrent VTE.
The incidence of symptomatic chronic thromboembolic pulmonary hypertension (CTEPH) in patients who have had an episode of PE varies according to the series and follow-up period, and has reached up to 3.8%.60 Some characteristics of the initial PE episode (age >70 years, young age, female gender, pulmonary systolic pressure >50mmHg, massive or sub-massive PE, recurrent or idiopathic PE) increase the risk of developing CTEPH.61
- •
In patients with a history of PE, it is recommended not to use thoracic imaging tests to evaluate the persistence of residual thrombosis or reperfusion of the initial defects.
- •
In patients with a history of PE and signs or symptoms suggestive of CTEPH, it is recommended to perform a follow-up transthoracic echocardiogram.
VTE is associated with an occult neoplasm in approximately 10% of patients. The Trousseau study62 compared the utility of extensive screening for cancer by thoracoabdominal CT and mammography (in women) with limited screening. The results did not show any differences in the incidence of cancer or mortality between the two patient groups. Extensive screening significantly increased the healthcare costs due to the additional diagnostic process associated with false positive results.
- •
In patients with an episode of unprovoked PE, it is suggested not to perform specific tests searching for neoplasia if there are no clinical symptoms or basic complementary examinations that suggest the presence of this disease.
Fernando Uresandi has received funding for giving lectures at educational events and/or scientific advice and/or research from Bayer HealthCare Pharmaceuticals.
Manuel Monreal has received funding for giving lectures at educational events and/or scientific advice and/or research from Bayer HealthCare Pharmaceuticals; Boehringer Ingelheim Pharmaceuticals, Inc.; Daiichi Sankyo, Inc; Pfizer; ROVI; Sanofi-Aventis.
Ferrán García-Bragado has received funding for giving lectures at educational events and/or scientific advice and/or research from Bayer HealthCare Pharmaceuticals; Boehringer Ingelheim Pharmaceuticals, Inc.; Bristol-Myers Squibb Company; Daiichi Sankyo, Inc; ROVI; Sanofi-Aventis.
Ramón Lecumberri has received funding for giving lectures at educational events and/or scientific advice and/or research from Bayer HealthCare Pharmaceuticals; Boehringer Ingelheim Pharmaceuticals, Inc.; Bristol-Myers Squibb Company; Leo Pharma; ROVI; Sanofi-Aventis.
Sonia Jiménez has received funding for giving lectures at educational events and/or scientific advice and/or research from Bayer HealthCare Pharmaceuticals; Boehringer Ingelheim Pharmaceuticals, Inc.; ROVI; Sanofi-Aventis.
Pedro Ruiz-Artacho has received funding for giving lectures at educational events and/or scientific advice and/or research from Bayer HealthCare Pharmaceuticals; LeoPharma; Sanofi-Aventis.
Francisco Lozano has received funding for giving lectures at educational events and/or scientific advice and/or research from Bayer HealthCare Pharmaceuticals; Bristol-Myers Squibb Company; Leo Pharma; ROVI; Sanofi-Aventis.
Antonio Romera has received funding for giving lectures at educational events and/or scientific advice and/or research from Bayer HealthCare Pharmaceuticals; Leo Pharma; ROVI; Sanofi-Aventis.
David Jiménez has received funding for giving lectures at educational events and/or scientific advice and/or research from Bayer HealthCare Pharmaceuticals; Boehringer Ingelheim Pharmaceuticals, Inc.; Bristol-Myers Squibb Company; Daiichi Sankyo, Inc; Leo Pharma; ROVI; Sanofi-Aventis.
Pere Domenech has received funding for giving lectures at educational events and/or scientific advice and/or research from Bayer HealthCare Pharmaceuticals; Boehringer Ingelheim Pharmaceuticals, Inc.; Bristol-Myers Squibb Company; Leo Pharma; ROVI; Sanofi-Aventis.
SEACV: Sergi Bellmunt, Jorge Cuenca, Álvaro Fernández, Fidel Fernández, Vicente Ibáñez, Francisco Lozano, José Ramón March, Antonio Romera; SEC: Luis Almenar, Antonio Castro, Pilar Escribano, María Lázaro, José Luis Zamorano; SEMES: José Ramón Alonso, José Ramón Casal, José Miguel Franco, Sonia Jiménez, Marta Merlo, Ramón Perales, Pascual Piñera, Pedro Ruiz-Artacho; Coral Suero; SEMI: Raquel Barba, Carmen Fernández-Capitán, Ferrán García-Bragado, Vicente Gómez, Manuel Monreal, José Antonio Nieto, Antoni Riera-Mestre, Carmen Suárez, Javier Trujillo-Santos; SEPAR: Francisco Conget, Luis Jara, David Jiménez, José Luis Lobo, Javier de Miguel, Dolores Nauffal, Mikel Oribe, Remedios Otero, Fernando Uresandi; SETH: Pere Domenech, José Ramón González-Porras, Ramón Lecumberri, Pilar Llamas, Eva Mingot, Elena Pina, Javier Rodríguez-Martorell.
General Coordinators: Fernando Uresandi and David Jimenez.
Please cite this article as: Uresandi F, Monreal M, García-Bragado F, Domenech P, Lecumberri R, Escribano P, et al. Consenso nacional sobre el diagnóstico, estratificación de riesgo y tratamiento de los pacientes con tromboembolia pulmonar. Arch Bronconeumol. 2013;49:534–547.
In this consensus document have participated the following scientific societies: Spanish Society of Pneumology and Thoracic Surgery (SEPAR); Society Española Internal Medicine (SEMI); Spanish Society of Thrombosis and Haemostasis (SETH); Spanish Society of Cardiology (ESC); Spanish Society of Medicine Accident and Emergency (SEMES); Spanish Society of Angiology and Surgery Vascular (SEACV).
- Home
- All contents
- Publish your article
- About the journal
- Metrics