Introduction
Before the appearance of low molecular weight heparins (LMWH), unfractionated heparins (UFH) were used to achieve anticoagulation during the acute phase of venous thromboembolism (VTE). The use of LMWH preparations can greatly simplify the treatment of VTE as administration is subcutaneous, at fixed dosage, and does not require laboratory monitoring except during pregnancy or in cases of renal insufficiency when anti-Xa activity should be measured. This, together with the fact that treatment can be followed at home, makes the cost/benefit ratio of LMWH superior to those of UFH.1
The efficacy and safety of LMWH in the treatment of deep vein thrombosis (DVT) has been demonstrated in a large number of studies. Some meta-analyses have even shown LMWH to be safer and more effective than UFH in this context.2 There is less scientific evidence regarding pulmonary thromboembolism (PTE), although studies have been published that show at least an equivalent level of safety and efficacy between LMWH and UFH.3-8 However, those studies have been carried out with different compounds (fraxiparine,3 dalteparin,4,8 reviparin,5 and tinzaparine6,7) and a variety of treatment regimens--once or twice a day--and the results obtained may not be true for each and every LMWH.9,10 We performed a multicenter, prospective, randomized trial to discover whether enoxaparin is as safe and effective as UFH in the initial treatment of thromboembolism.
Patients and methods
This was a multicenter, prospective open study, where enoxaparin (1 mg/kg every 12 hours) was compared with UFH in the treatment of submassive PTE. The protocol included objective diagnosis of PTE and DVT and was approved by the Ethics Committee of the Galician Public Health Service (SERGAS). The study was carried out in 3 Spanish hospitals: the Hospital XeralCalde in Lugo, the Hospital Comarcal in Monforte, and Complejo Hospitalario in Pontevedra.
The patients enrolled in the study were over 18 years old, were diagnosed with PTE, gave written informed consent, and had none of the following exclusion criteria: previous episode of DVT, PTE with hemodynamic repercussion, known factor of hypercoagulability, anticoagulant treatment, pregnancy, formal contraindication for anticoagulation or serious concomitant illnesses.
The patients were randomized from the lists of enrolled patients at each center using the SAS statistics program. All patients underwent a ventilation-perfusion scan within 24 hours and plethysmography of the lower extremities. Intraluminal filling defect in 2 or more projections or a sudden cutoff in a section of the deep vein system was considered proof of DVT. A PTE diagnosis was accepted if the results of the ventilation-perfusion scan were classified as "highly probable" following the Prospective Investigation of Pulmonary Embolism Diagnosis criteria11 or if the arteriography was positive. Arteriography results were only considered positive if there was presence of an intraluminal filling defect in 2 projections and evidence of occlusion of a pulmonary artery. A PTE diagnosis was also accepted in patients with positive plethysmography and compatible conditions without evidence of other pulmonary disease that could cause the symptoms although the results of the scan were not "highly probable." If the scan results were normal, a PTE diagnosis was ruled out.
Patients were assigned to 1 of 2 treatment groups: Group A who were administered enoxaparin, 1 mg/kg weight every 12 hours, and Group B, who were administered 5% sodium heparin (Laboratorios Farmacéuticos ROVI, Madrid, Spain) and received an initial bolus dose of 5000 IU through an infusion pump (IVAC 5910®, IVAC CORP, San Diego, CA, USA), which was adjusted according to the partial thromboplastin time results to an approximate dose of 35 000 IU /day. When diagnosis of PTE was confirmed and no other diagnostic tests were needed, oral anticoagulation with acenocoumarol was started. Both treatments were continued simultaneously until an international normalized ratio (INR) of 23 was reached. Controls were programmed at 1, 3, and 6 months after hospital discharge to examine the clinical evolution and evaluate whether anticoagulation was sufficient (INR >2 in over 70% of controls). Patients were instructed to report to the hospital at any sign of a new episode of DVT or PTE. Recurrence was considered confirmed if the perfusion scan showed perfusion defects that had not existed in the initial exploration, if the arteriography showed new intraluminal filling defects, if the plethysmography showed a new venous region affected, or if there was a proximal thrombus extension of over 5 cm. Bleeding was considered "major" when it was intracranial, retroperitoneal, needed transfusion or when hemoglobin decreased by 2 or more points.
Statistical analysis
The mean and standard deviation and percentage for qualitative variables were used for the descriptive statistical analysis. Continuous variables of the patients of the 2 groups were compared using the Student t test or the MannWhitney test depending on the distribution of the variable. Qualitative variables were compared between the groups using the Fisher´s exact test.
Results
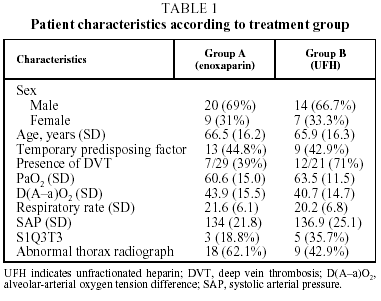
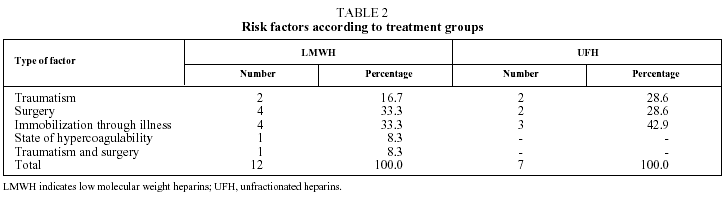
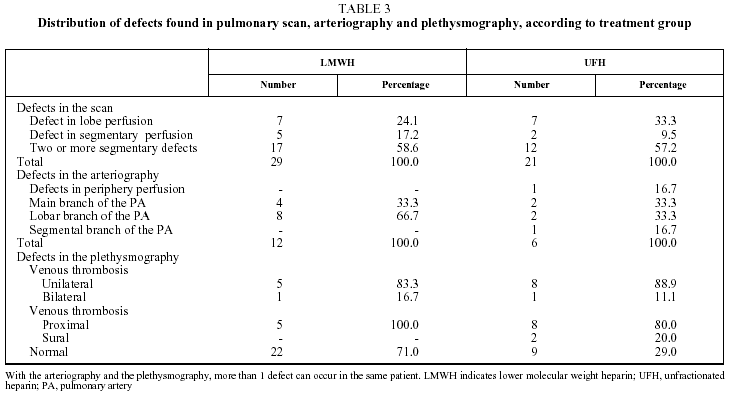
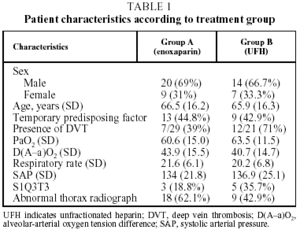
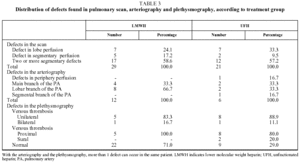
Fifty-six patients with PTE were enrolled in the study. Six patients were withdrawn, 2 for incorrect diagnosis, 2 for antecedents of venous thromboembolism, and 2 more were lost to follow-up. The 50 remaining patients (34 men and 16 women with a mean [SD] age of 66.2 [16.1] years), were assigned to receive enoxaparin (Group A, n=29) or UFH (Group B, n=21). The clinical characteristics of the study population are summarized in Table 1. No significant differences between the groups were found for age (P=.783), sex (P>.999), presence or absence of predisposing factor (P>.999), respiratory rate (P=.286), systolic blood pressure (P=.661) electrocardiogram abnormalities (P=.768), PaO2 (P=.710) or radiological irregularities (P=.252). Predisposing factors are shown for both treatment groups in Table 2. In Group A, 29% of patients were diagnosed with DVT, compared with 71% in Group B, which was a significant difference (P=.030). Arteriographies had to be performed on 10 patients from Group A and 6 from Group B. The location and extent of the defects found in the scan, arteriography, and plethysmography are shown in Table 3.
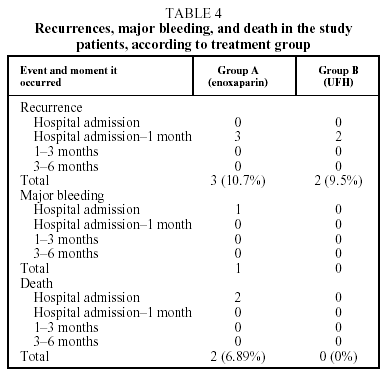
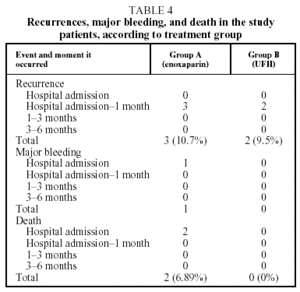
Recurrent thromboembolism was diagnosed in 3 patients from Group A (10.7%) and 2 patients from Group B (9.5%) (Table 4). No significant differences were found between the 2 groups (P=.878). The recurrence was pulmonary in all cases and occurred between hospital discharge and the first revision 1 month later. No patient died from a recurrence. Recurrence coincided with anticoagulation below the therapeutic range (INR<2) in only 1 case, which was from group B.
Two patients died, both from Group A. One death was caused by pulmonary aspergillosis and occurred during the initial hospitalization. The other occurred 7 days after enrollment in the study, from hypovolemic shock attributed to a massive retroperitoneal hemorrhage secondary to the oral anticoagulation (Table 4). This was the only episode of major bleeding recorded throughout the study. Table 4 details the number of recurrences, hemorrhages and deaths in both groups during the study.
Mean time of hospitalization was 10.4 days in Group A and 10.2 days in Group B (P=.903).
Discussion
Despite the fact that LMWH are replacing UFH for the initial treatment of DVT, they are compounds of different molecular weights, with different affinities for plasma proteins, different plasma half-lifes and varying factor Xa and thrombin inhibitory activities. Our study compared the efficacy and safety of enoxaparin and UFH in the initial treatment of submassive PTE. Patients were divided into 2 groups according to the initial treatment. The groups were comparable in age, sex, presence or absence of a temporary risk factor (immobilization), and PaO2 on enrollment. Our study (in which the mean age was 66 years) enrolled significantly more men than women, a pattern which is not in keeping with the findings of Stein et al12 who found that among patients aged over 50 years the incidence was higher for women. It should also be pointed out that there was a significantly larger number of patients with DVT in the UFH treatment group, probably by chance. Some studies indicate an increased risk of recurrence in patients with proximal DVT at the moment treatment starts,13 but our study did not.
Five episodes of recurrence were recorded in our study: 3 in the enoxaparin group and 2 in the UFH group. The patients concerned (4 women and 1 man) had a mean age of 66 (range 4480), and in 4 of the 5 cases there was a prior predisposing factor (surgery or injury). This notable prevalence of temporary risk factors differed from the published data,14 where the subgroup of patients with such risk had a lower percentage of recurrence (1.4%) with respect to the subgroup of idiopathic VTE patients (6.7%). Our results offer a similar recurrence percentage for both treatment groups: 10.7% for Group A and 9.5% for Group B (P=.878). All the recurrences were pulmonary, happened between the initial hospitalization and the first revision (1 month), and were diagnosed according to strict criteria. In all cases patients were receiving oral anticoagulation and no recurrence caused death. These percentages are higher than those published in the Columbus study5 (5.9% of recurrence with reviparin), in the Simmoneau et al study6 (1.6% with tinzaparin), and in the Hull et al study7 (no recurrence with tinzaparin) and is among the highest found in other studies.15,16 The recurrences cannot have been due to inadequate oral anticoagulation (INR<2) as this only occurred in 1 patient. Length of treatment (47 days) with enoxaparin or UFH was similar to usual regimens and does not seem to have affected recurrence, and it is unlikely that the use of enoxaparin to treat PTE could have been the cause as the percentage of recurrence was not significantly different with enoxaparin and UFH treatment.
While it is true that the use of LMWH reduces costs, avoiding hospitalization or shortening hospital stays,1,8 we did not find significant differences in the latter, given that our study was designed to have 2 treatment groups with similar characteristics.
The main limitation of this study was the number of patients enrolled, too few to make definite conclusions. However, the results agree with those already published and we consider enoxaparin to be a safe, effective initial treatment for patients with PTE.
This study was presented at the 12th Annual Congress of the European Respiratory Society (Stockholm, 2002).
Correspondence: Dr. L.A. Pérez de Llano.
Unidad de Endoscopias. Hospital Xeral de Lugo.
Severo Ochoa, s/n. 27004 Lugo. Spain.
Manuscript received November 7, 2002.
Accepted for publication February 4, 2003.