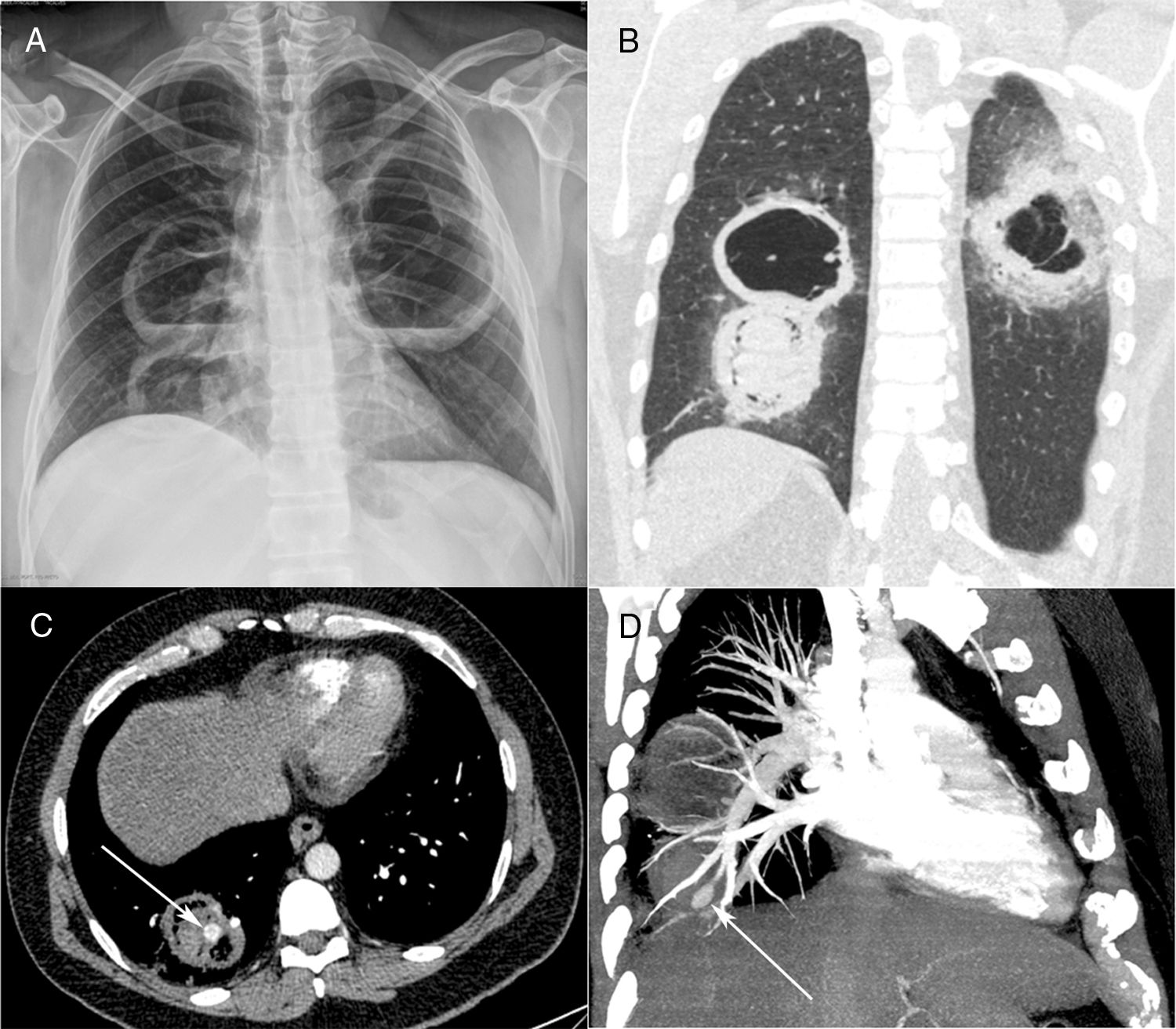
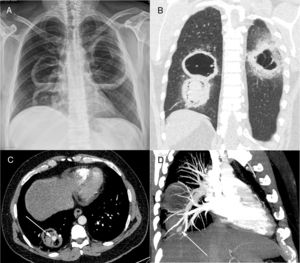
A 40-year-old diabetic man with 40 pack-years smoking exposure presented to the emergency department with a 30-day history of fever, dyspnea, and productive cough with thick yellowish sputum, later associated with hemoptysis. The patient had type 2 diabetes mellitus and reported unmeasured weight loss over the last month. Laboratory tests revealed leukocytosis (18,500cells/mm3); the patient's blood glucose level was 296mg/dL and his glycosylated hemoglobin concentration was 12.8%. His basal oxygen saturation was 89%. Other laboratory findings were unremarkable. A chest-X ray showed three cavitary lesions with air-fluid levels (Fig. 1A). The patient's sputum was negative for acid-fast bacilli.
Posteroanterior chest radiograph (A) showing three large cavitary lesions, two on the right lung and one on the left, with air-fluid levels. Coronal computed tomography image (B) obtained with the lung window setting demonstrating the thick-walled cavitary lesions, with solid content in one lesion on the right. Axial contrast-enhanced image (C) obtained with the mediastinal window setting depicting the solid content in the lower cavity, with a highly enhancing nodule inside (arrow), compatible with an aneurysm. Computed tomography–pulmonary angiography image (D) demonstrating that the pseudoaneurysm is related to a peripheral pulmonary artery (arrow).
Contrast-enhanced chest computed tomography (CT) disclosed thick-walled cavitary lesions, one with solid content and a pseudoaneurysm inside, located on a peripheral ramification of the right pulmonary artery (Fig. 1B–D). Fiberoptic bronchoscopy revealed necrotic material covering the right main bronchial mucosa. Bronchoalveolar lavage (BAL) demonstrated the presence of fungal hyphae suggestive of zygomycosis. Cultures confirmed the presence of Rhizopus species. The patient was treated with amphotericin B. During hospitalization, he had several episodes of hemoptysis, and he died of massive hemoptysis on the second day after the internation admission.
Mucormycosis (also known as zygomycosis) is a fulminant opportunistic fungal infection caused by fungi of the order Mucorales, class Zygomycetes. Mucormycosis is far less common than other opportunistic fungal infections, such as Aspergillus and Candida infections, although the mortality rate is much higher. Although Classically described in patients with diabetes (especially diabetic ketoacidosis), in the modern era it is seen most commonly in patients with hematological malignancies, particularly those who have undergone stem cell transplantation.1–3 Lin et al.4 described 35 patients with confirmed pulmonary mucormycosis; hematological malignances were found in 68% and diabetes in 20% of the cases.
Mucormycosis manifests in the sinuses (39%), lung (24%), skin (19%), brain (9%), and gastrointestinal tract (7%), and as disseminated disease (6%). The respiratory symptoms of pulmonary mucormycosis are diverse, including fever, cough, expectoration, chest pain, and breathing difficulties.1,2 Mucorales species are angioinvasive, which leads to vessel rupture with massive hemorrhage and infarction of the distal tissue. Hemoptysis is a common complication and can be massive, causing sudden death.1 The clinical diagnosis of mucormycosis is difficult, and is often made at a late stage or postmortem. The diagnosis relies on the identification of organisms in tissues by pathological examination, with confirmation by culture.1,2,5 However, some authors have reported, as in our case, initial diagnosis by identification of the fungus in sputum or BAL fluid, with subsequent confirmation by culture. In one case series, 25% of sputum or BAL specimens allowed positive identification.6 The main differential diagnosis is pulmonary aspergillosis. On pathological examination, hyphae characteristics differ between Mucor and Aspergillus species. The hyphae of Mucor species are thick and non-septate, and have larger diameters than the hyphae of Aspergillus (which have fewer, irregular branches and form right or obtuse angles).2
Most patients with pulmonary mucormycosis show consistent sequential morphological changes on serial follow-up imaging, consisting initially of consolidation or nodules/masses with the CT halo sign, followed by the reversed halo sign or central necrosis and, finally, the air-crescent sign. These changes are related to recovery of the absolute neutrophil count.3Aneurysms affecting the pulmonary arteries are extremely uncommon. The most common cause of this complication is infection. Other causes are pulmonary hypertension, pulmonary arteritis, cystic medial necrosis, and thoracic trauma. The most common organisms are bacteria. Fungal invasion of pulmonary artery walls, resulting in pseudoaneurysm formation, is exceedingly rare.1,7,8 Physicians should consider pulmonary mucormycosis in immunocompromised patients with unresolved pneumonia. In addition, the high fatality rates associated with pulmonary artery pseudoaneurysm warrant aggressive management with surgery and antifungal agents.