Some pro-inflammatory lipids derived from 1 lipooxygenase enzyme are potent neutrophil chemoattractant, a cell centrally involved in acute respiratory distress syndrome (ARDS); a syndrome lacking effective treatment. Considering the beneficial effects of the leukotriene receptor inhibitor, montelukast, on other lung diseases, whether montelukast attenuates inflammation in a mouse model of ARDS, and whether it reduces LPS stimulated activation of human neutrophils was investigated.
MethodsThirty-five C57Bl/6 mice were distributed into control (PBS)+24h, LPS+24h (10μg/mouse), control+48h, LPS+48h, and LPS 48h+Montelukast (10mg/kg). In addition, human neutrophils were incubated with LPS (1μg/mL) and treated with montelukast (10μM).
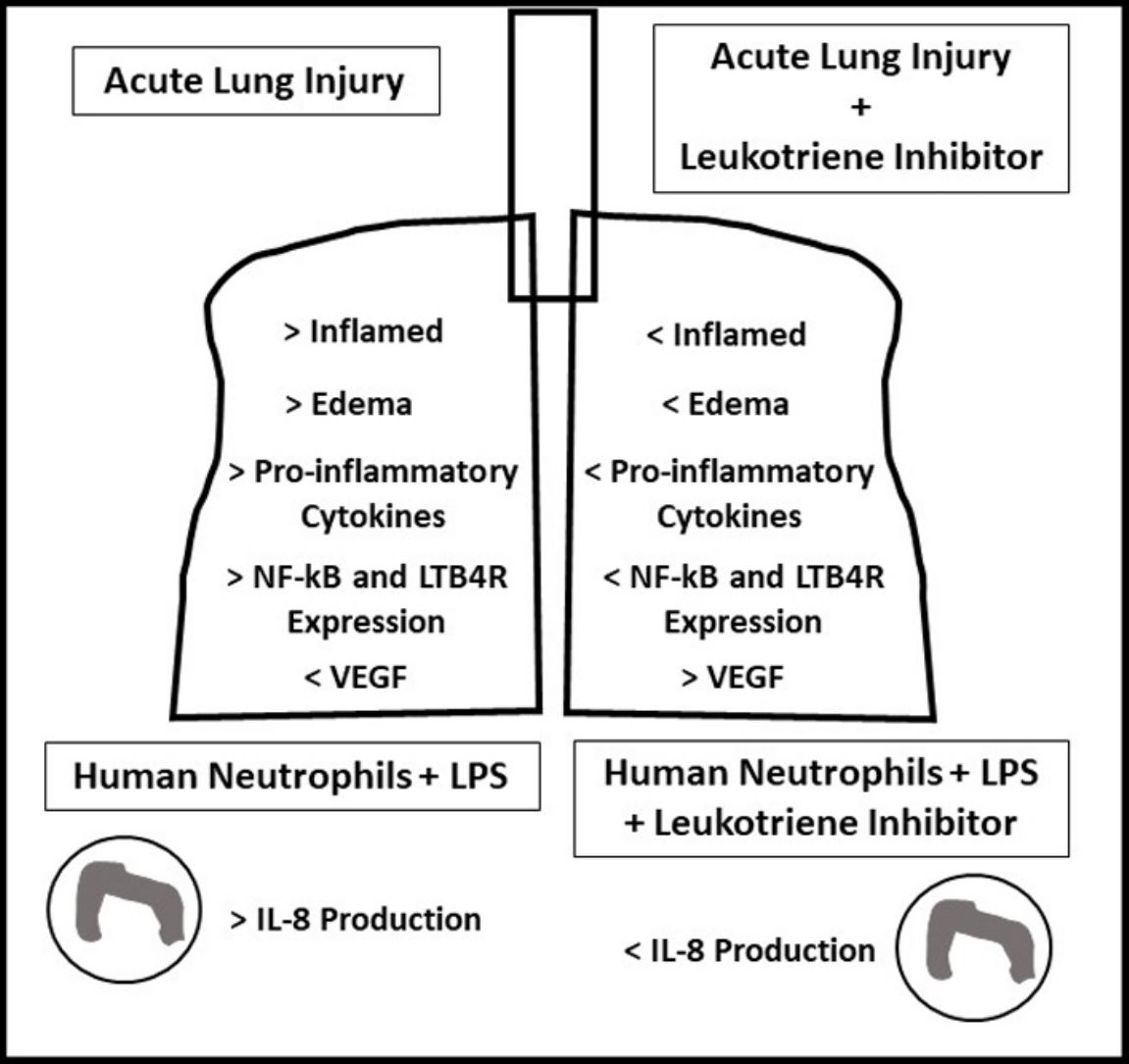
ResultsOral-tracheal administration of montelukast significantly attenuated total cells (P<.05), macrophages (P<.05), neutrophils (P<.01), lymphocytes (P<.001) and total protein levels in BAL (P<.05), as well as IL-6 (P<.05), CXCL1/KC (P<.05), IL-17 (P<.05) and TNF-α (P<.05). Furthermore, montelukast reduced neutrophils (P<.001), lymphocytes (P<.01) and macrophages (P<.01) in the lung parenchyma. In addition, montelukast restored BAL VEGF levels (P<.05). LTB4 receptor expression (P<.001) as well as NF-κB (P<.001), a downstream target of LPS, were also reduced in lung parenchymal leukocytes. Furthermore, montelukast reduced IL-8 (P<.001) production by LPS-treated human neutrophils.
ConclusionIn conclusion, montelukast efficiently attenuated both LPS-induced lung inflammation in a mouse model of ARDS and in LPS challenged human neutrophils.
Algunos lípidos proinflamatorios derivados de la enzima lipooxigenasa 1 son potentes quimioatrayentes de neutrófilos, un tipo celular con una implicación principal en el síndrome de distrés respiratorio agudo (SDRA), para el que no hay tratamiento efectivo. Considerando los efectos beneficiosos del inhibidor de los receptores de leucotrienos montelukast en otras enfermedades pulmonares, se investigó si este fármaco era capaz de atenuar la inflamación en un modelo de ratón de SDRA y de reducir la activación de los neutrófilos humanos inducida por LPS.
MétodosSe utilizaron 35 ratones C57BL/6 distribuidos en los siguientes grupos: control (PBS)+24h, LPS+(24h [10μg/ratón]), control+48h y LPS 48h+montelukast (10mg/kg). Por otro lado, se incubaron neutrófilos humanos con LPS (1μg/ml) y se trataron con montelukast (10μM).
ResultadosLa administración orotraqueal de montelukast redujo el número total de células (p<0,05), de macrófagos (p<0,05), de neutrófilos (p<0,01), de linfocitos (p<0,001) y los niveles totales de proteína en el lavado broncoalveolar (p<0,05), así como de IL-6 (p<0,05), CXCL1/KC (p<0,05), IL-17 (p<0,05) y TNF-α (p<0,05). Además, el montelukast redujo los neutrófilos (p<0,001), los linfocitos (p<0,01) y los macrófagos (p<0,01) en el parénquima pulmonar. Asimismo, restauró los niveles de VEGF en el lavado broncoalveolar (p<0,05) y disminuyó la expresión del receptor LTB4 (p<0,001) y de NF-κB (p<0,001), una diana downstream del LPS, en los leucocitos del parénquima pulmonar. Por último, redujo la producción de IL-8 por parte de los neutrófilos humanos tratados con LPS.
ConclusiónEn conclusión, el montelukast atenuó de manera eficaz tanto la inflamación pulmonar inducida por LPS en un modelo de ratón de SDRA como en neutrófilos humanos estimulados con LPS.
Acute respiratory distress syndrome (ARDS) is characterized by hypoxemic respiratory failure associated with acute pulmonary inflammation and edema presenting high mortality rates.1 Different etiologies, such as head, chest and other major injuries, as well as sepsis, inhalation of harmful substances and severe pneumonia may result in the development of ARDS.1–3 Although the mechanisms underlying the pathophysiology of ARDS are not completely understood, in all cases; especially in bacterial infections, an exacerbated inflammatory response plays a central role.1–3
While several animal models have been established to study ARDS, lipopolysaccharide (LPS) is the most widely used as it reproduces several important ARDS features, such as the accrual of neutrophils in alveolar and in interstitial space and in bronchoalveolar lavage (BAL), the accumulation of proteinaceous debris in alveolar spaces, thickening of the alveolar wall, and increased concentration of total proteins and pro-inflammatory cytokines in BAL.2–5
LPS signals via toll like receptor 4 (TLR4) and activates nuclear factor kappa B (NF-κB), a key regulator of the inflammatory process.3–6 NF-κB is a transcription factor regulating several aspects of ARDS pathophysiology, such as production of pro-inflammatory cytokines (i.e. IL-1beta, IL-6, IL-8/CXCL-1 and TNF-α), and also the lung fibroproliferative response in murine models of ARDS.7–10 NF-κB also regulates human and mouse fibroblast differentiation,11 a key event that occurs during fibroproliferation, an important process following lung injury. Therefore, pharmacological approaches that inhibit NF-κB expression and activation may attenuate lung inflammatory and fibrotic responses following injury.3–5,10–12
Cys leukotrienes receptor-1 (cysLTR1) antagonists montelukast, pranlukast and zafirlukast are small molecules that have demonstrated secondary, off-target, anti-inflammatory effects including the inhibition of cyclic nucleotides phosphodiesterases and 5′-lypoxygenase as well as NF-κB downregulation.13
Taken together, this study tested the hypothesis that montelukast inhibits both acute lung injury induced by LPS in mice and LPS-induced human neutrophil activation.
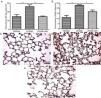
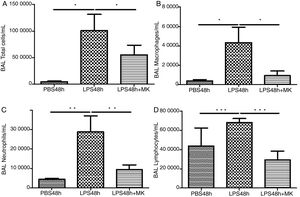
ResultsMontelukast Reduces Leukocyte Number in Bronchoalveolar Lavage (BAL)Similarly to the leukocyte response during ARDS, oral-tracheal administration of LPS results in a significant increase in the number of lung leukocytes. At 24h, BAL leukocytes were significantly increased in mice injured with LPS (Fig. 1A). To mimic an ARDS therapeutic scenario, mice received oral-tracheal administration of montelukast 24h post-LPS injury and were sacrificed 24h later. At 48h, total number of BAL cells remained significantly increased, however, montelukast significantly reduced the number of cells in the BAL (Fig. 1B). At 24h post-LPS treatment, differential cell counts of BAL fluid revealed a significant increase in the number of macrophages, neutrophils and lymphocytes compared to PBS treated controls (Fig. 1C, E, G). Montelukast treatment at 24h, significantly attenuated the accumulation of macrophages (Fig. 1D), neutrophils (Fig. 1F), and lymphocytes at 48h (Fig. 1H).
Therapeutic administration of montelukast reduced leukocyte number in bronchoalveolar lavage (BAL). Results are expressed as mean±SEM. For (A and B) * P<.05 compared with PBS48h and PBS48h+MK group. For (C) **P<.01 compared with PBS48h and LPS48h+MK group. For (D) *** P<.001 compared with PBS48h group and LPS48h+MK group.
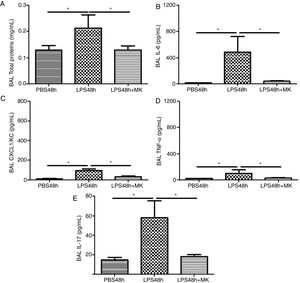
Compared to PBS treated mice, increased vascular permeability was evident by increased protein concentration in BAL fluid at 24h post-LPS treatment (Fig. 2A) and remained at 48h (Fig. 2B), while Montelukast treatment at 24h post-LPS reduced the total amount of BAL proteins (Fig. 2B). At 24h post-LPS treatment, ELISA detected increased levels of proinflammatory cytokines IL-6 (Fig. 2C), CXCL1/KC (Fig. 2E), TNF-α (Fig. 2G) and IL-17 (Fig. 2I). At 48h post-LPS treatment, cytokines remained high: IL-6 (Fig. 2D), CXCL1/KC (Fig. 2F), TNF-α (Fig. 2H) and IL-17 (Fig. 2J), but were effectively reduced by therapeutic administration of Montelukast.
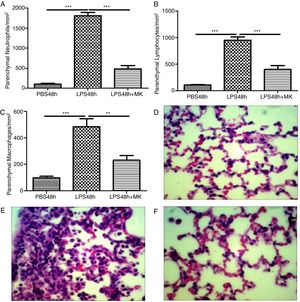
Montelukast Reduces Neutrophils, Lymphocytes and Macrophages Accumulation in the Lung ParenchymaIndeed, at 24 and 48h post-LPS treatment, morphometric analysis revealed increased numbers of parenchymal neutrophils (Fig. 3A, B), lymphocytes (Fig. 3C, D), and macrophages (Fig. 3E, F). Treatment with Montelukast significantly reduced the number of immune cells in the lung parenchyma (Fig. 3B, D, F). Representative images of PBS (Fig. 3G), LPS+24h (Fig. 3I), LPS+48h (Fig. 3H), and 96 LPS+Montelukast (Fig. 3J).
Therapeutic administration of montelukast reduced neutrophils, macrophages and lymphocytes in the lung parenchyma. Results are expressed as mean±SEM. For (A and B) *** P<.001 compared with PBS48h and LPS48h+MK group. For (C) *** P<.001 compared with PBS48h and ** P<.01 compared with PBS48h+MK group. (D, E and F) are representative photomicrographs of HE lung stained slides in PBS48h, LPS48h and LPS48h+MK groups, respectively.
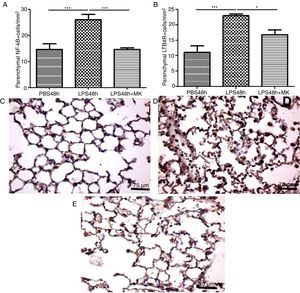
In the present study, increased expression of NF-κB by parenchymal leukocytes for both periods studied (24h and 48h) post-LPS stimulation compared with PBS groups (Fig. 4A and B) was observed. The results also revealed that Montelukast efficiently reduced the NF-κB expression (Fig. 4B). LTB4 signals mainly via LTB4R, which results in the recruitment of neutrophils. The present study demonstrated that LPS stimulation at 24h and 48h resulted in increased expression of LTB4R by parenchymal leukocytes (Fig. 4C and D) while Montelukast significantly reduced the LTB4R expression (Fig. 4D). Fig. 4E–H shows representative photomicrographs of NF-kB for PBS48h, LPS24h, LPS48h and LPS48h+montelukast groups, respectively.
NF-kB and LTB4R expression by leukocytes in lung parenchyma were reduced by therapeutic montelukast administration. Results are expressed as mean±SEM. For (A) *** P<.001 compared with PBS48h group and LPS48h+MK group. For (B) *** P<.001 compared with PBS48h and * P<.05 LPS48h+MK group. (C, D and E) are representative photomicrographs of immunohistochemistry for NF-kB in PBS48h, LPS48h, LPS48h and LPS48h+montelukast groups, respectively.
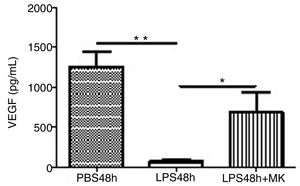
Treatment with LPS decreased VEGF expression at 24h (Fig. 5A) and furthermore at 48h while Montelukast administration at 24h resulted in a recovery of VEGF expression in BAL compared to control (PBS) treated animals (Fig. 5B).
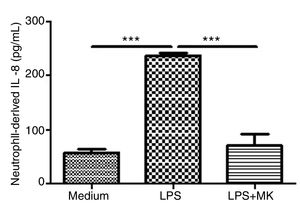
Montelukast Reduces IL-8 Release by Human NeutrophilsHuman neutrophils were stimulated with LPS (1.5μg/mL) for 1h and then incubated with Montelukast (10μM in PBS) for an additional 5h. ELISA for IL-8 was performed on the supernatant. Montelukast treatment significantly inhibited LPS-stimulated production of IL-8 (Fig. 6A).
Montelukast suppressed IL-8 levels production by human neutrophils stimulated with LPS. IL-8 levels in neutrophil supernatant were measured using ELISA. The results are expressed as mean±SEM. For this figure *** P<.001 compared with non-stimulated (Medium) and LPS-stimulated and treated with montelukast (LPS+MK group).
The present study demonstrates for the first time that leukotriene inhibitor Montelukast reduces both acute LPS-induced lung inflammation in mice as well as LPS-induced human neutrophils activation. Exacerbated inflammation plays a central role in the pathogenesis of ARDS; therefore in the quest to develop effective ARDS therapies, reducing inflammation has been a main goal. The off-target effects of Montelukast on LTB4 receptors present on neutrophils could have beneficial implications in ARDS therapy. Leukotriene B4 (LTB4) is synthesized primarily by activated basophils, eosinophils, monocytes and macrophages and acts in both an autocrine and paracrine manner by signaling to structural cells, neutrophils and TH2 lymphocytes.14 Though neutrophils express a low level of LTB4 receptor, LTB4 acts as a strong chemoattractant of neutrophils, a cell centrally involved in ARDS.
While the recognition of the involvement of leukotriene pathways in asthma prompted the development of leukotriene receptor inhibitors, drugs such as Montelukast, a leukotriene inhibitor, have not yet been tested in the context of ARDS.15 Twenty-five years ago, a swine LPS model of acute lung injury (ALI) demonstrated that the LTBR1 competitive receptor antagonist LY255283 reduced ALI.16 However, unlike Montelukast, this agent did not exhibit potent off target anti-inflammatory effects such as the inhibition of cyclooxygenase or 5-lipoxygenase enzymes.17 Therefore, in the case of ARDS, Montelukast may be an effective inhibitor of inflammation not only because of its ability to inhibit leukotriene signaling, but also via off-target effects which result in a net increase in cyclic adenosine monophosphate (cAMP),18 and the suppression of NF-kB19 which leads to the attenuation of cytokine production and a dispersal of lung immune cells.
This study used the LPS-induced acute lung injury model in mice to test the hypothesis that the Montelukast would attenuate lung inflammation. Our results indicated that Montelukast reduced the LPS-induced increase in total immune cells both in the BAL, suggesting decreased vascular permeability, and in the lung parenchyma. In addition, Montelukast treated mice displayed significantly reduced levels of cytokines IL-6, CXCL1/KC, IL-17 and TNF-α suggesting attenuation of inflammatory processes due to LPS. LPS-induced pulmonary dysfunction was associated with increased neutrophil count, leukotriene (LT) B4, and tumor necrosis factor (TNF)-α in BALF. These results suggest that treatment with Montelukast can be useful in chronic airway inflammatory diseases including COPD poorly responsive to glucocorticoids.20 In addition, Montelukast treated mice also displayed reduced LTB4R and NF-κB expression in the lung parenchyma, which correlates to decreased leukotriene signaling and decreased inflammation and Montelukast was able to block LTD(4)-induced stimulation. Allergen challenge leads to a significant increase in sCD14 concentrations in BAL and might modulate the allergen-induced inflammation. In addition, LTD(4) might play a role in the release of sCD14, and it could be speculated that sCD14 reduction by LTRA might contribute to the mechanisms of LTRA in the treatment of allergic asthma.21 Moreover, LTB(4)- and LTD(4)-induced eosinophil activation was attenuated by CP-105,696 and the Cys-LT(1) receptor antagonist montelukast, respectively, highlighting specific receptor dependency.6 Thus, mediator-triggered granulocyte activation and antiapoptotic pathways are distinct events that can be differentially regulated.22 Lastly, our results presented herein indicates that Montelukast treatment resulted in a recovery of VEGF expression in the BAL, a measurement which is associated with recovery in ARDS patients.23–25
While neutrophils themselves do not produce LTB4, and LTB4 receptors are expressed only at low levels, exposure to LTB4 primes neutrophils to produce copious amounts of reactive oxygen species (ROS), matrix metalloproteinases (MMPs) and other cytokines upon stimulation by additional cytokines. Human neutrophils activated with the chemoattractant N-formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP) in combination with cytochalasin B resulted in abrupt and sustained increases in cytosolic Ca2(+), as well as release of elastase and production of superoxide and LTB4, and expression of macrophage complement receptor 3 (CR3). These effects were attenuated with Montelukast treatment and the literature point out its association with significant increases in cyclic AMP.26 In this study, human neutrophils were stimulated with LPS which signals via TLR4 and activates NF-kB-signaling resulting in the production of cytokines, particularly IL-8.27 Stimulation with LPS for 1h, followed by one treatment of Montelukast resulted in a significant reduction of IL-8 in the cell supernatant. Given the low level of LTBR4 receptor expression by neutrophils, this study also suggests that an important off-target effect of Montelukast is the ability to attenuate NF-kB signaling, likely through upstream mechanisms that increase intracellular cAMP.
An interesting point that should be highlighted is the fact that our results showed herein a reduction of circulating levels of IL-17 in Montelukast-treated mice with acute lung inflammation. Therefore, from our results, is possible suggest that the Th17 cells could be a bystander mediator of Montelukast effect in vivo, since that IL-17 is produced by Th17 cells, and this subset expresses high levels of LTB4R1 and CysTLR1, being attracted by leukotrienes (especially LTD4).28
In conclusion, this is the first study to report the effects of Montelukast in an LPS mouse model of ARDS and in LPS-stimulated human neutrophils activation. These results concur not only the potent anti-inflammatory nature of Montelukast but also its ability to signal via LTB4R independent pathways. Future studies should further explore the nature of these mechanisms especially in the context of human neutrophils, a central inflammation mediator of ARDS.
Material and methodsExperimental Design of Animal ExperimentsThis study was approved by the ethical committee of Nove de Julho University (AN0002/2015) and were carried out in accordance to Guide for the Care and Use of Laboratory Animals, published by the U.S. National Institutes of Health (NIH publication no. 85-23, revised 1996). Thirty-eight C57Bl/6 male mice weighing between 20 and 25g were distributed in Control 24h (PBS24h; n=7), LPS 24h (LPS24h; n=7), Control 48h (PBS48h; n=8), LPS 48h (LPS48h; n=8) and LPS 48h+montelukast (LPS 48h+MK; n=8). For Montelukast administration, animals were anesthetized by intra-peritoneal injection of ketamine (100mg/kg) and xylazine (10mg/kg). The PBS24h and LPS24h groups were euthanized 24h after vehicle (PBS 50μl) or LPS (10μg/mouse/50μl PBS) administration. The PBS48h, LPS48h and LPS 48h+MK groups were euthanized 48h after vehicle (PBS; 50μl) or LPS (10μg/mouse/50μl PBS) administration. Montelukast (10μM/mouse/50μl PBS) was orotracheally administered 24h after LPS administration and animals were euthanized 24h later.
Assessment of Lung InflammationLung inflammation was assessed through the collection and analysis of bronchoalveolar lavage (BAL). Quantitative analysis of parenchymal inflammation was performed using histomorphometrical technique.29–31
The numbers of total and differential cells in BAL were evaluated in the material recovered from 3 gentle washes of 0.5mL of sterile PBS, by using a Neubauer chamber (total cells) and cytospin preparations (differential cell count).29–31 The cytospins were stained with Diff Quick (Medion Diagnostics, Düdingen, Switzerland), as previously described.27–32 In summary, 300cells per slide per mouse were analyzed using an optical microscope and were counted according to the classical hematological criteria.29–34
The density of neutrophils, lymphocytes and macrophages in the lung parenchyma was evaluated as previously described.29–31 Briefly, the lungs were excised in block, fixed in 10% formalin solution, at a constant pressure (20cmH2O) for 24h, and submitted to histological routine.33
Five micrometer lung slices were stained with hematoxylin and eosin and 20 photomicrographs at 400× magnification of each animal of all groups were obtained using an Olympus BX43-L-FL microscope with a camera XM-10. The area of lung tissue in the lung parenchyma was obtained by subtracting the airspace area from the total photo area. Then, the number of neutrophils, lymphocytes and macrophages were counted in each photo according to the morphological criteria. The results were expressed in number of cells/mm2 of lung tissue.30–33
Total Proteins in BALThe levels of total proteins in BAL was measured using the BCA Protein Assay Kit (Thermo Scientific, USA) and was used as an index of vascular permeability.31
Cytokines in BAL, Serum and in Cell Culture SupernatantsThe levels of IL-6 (Biolegend, Code 431306; Detection limit 7.8pg/mL), CXCL1/KC (R&D Systems, Code DY453; Detection limit 15.6pg/mL), IL-8 (Biolegend, Code 431506; Detection limit 15.6pg/mL), IL-17 (R&D Systems, Code DY421; Detection limit 15.6pg/mL) and TNF-α (Biolegend, Code 430906; Detection limit 7.8pg/mL) in BAL and in cell culture supernatants were measured using ELISA kits from Biolegends (USA) and R&D Systems (USA) as indicated, according to the manufacturers’ recommendations.33 The measurements were done in triplicates in all samples.
Immunohistochemical StudyThe quantitative analysis of the expression of leukotriene B4 receptor (LTB4R) (sc-98841; Santa Cruz, CA, USA) and of NF-κB p65 (sc-109; Santa Cruz, CA, USA) by parenchymal leukocytes was performed using classical immunohistochemistry protocol, as previously described.30,33,34 Briefly, the area of lung tissue in the lung parenchyma was obtained by subtracting the airspace area from the total photo area. Then, the number of leukocytes positive to LTB4R and NF-kB p65 were counted in each photo. The results were expressed in number of positive cells/mm2 of lung tissue.29–32
Isolation and Culture of Human NeutrophilsEight milliliter of peripheral blood was diluted 1:1 in sterile PBS and added to a tube containing Ficoll Paque gradient and submitted to 20min centrifugation (1200×g). Red cells were lysed, polymorphonuclear cells collected and neutrophils were separated from eosinophils using EasySep™ Human Neutrophil Enrichment Kit (#19257; StemCell Technologies, USA). The purity was assessed through cytospin analysis and was higher than 98%. Neutrophils (1×106/2mL medium/well) were incubated in 48 well plates in RPMI 1640 medium and were stimulated with LPS (1.5μg/mL medium) for 1h and then incubated with montelukast (10μM in PBS) for an additional 5h. Supernatant was recovered for measurements of IL-8 by ELISA.
Statistical AnalysisIf not stated otherwise, two-way analysis of variance (TWO-WAY ANOVA) followed by Bonferroni post hoc test was used. Significance levels were considered for P<.05. Values were expressed as mean±SEM.
AuthorshipJEDC, MCOJ, BM, AD, ARAO, JCJAJ, AAB, NCRO, NRDR, contributed performing the experiments and analysis. APLO, APS, FMCC, FA, HCCFN, and RPV have written the manuscript and critically revised the manuscript and performed the statistical analysis. RPV have designed the study. All authors have reviewed and approved the final version of the manuscript prior to submission.
This study was supported by Sao Paulo Research Foundation (FAPESP), grant 2012/15165-2. MCOJ holds a PhD fellowship from FAPESP (2014/14604-8). BM holds a postdoctoral fellowship from FAPESP (2014/23196-0). ARAO holds a MSc fellowship from FAPESP (2014/07500-1). JCJAJ holds a MSc fellowship from FAPESP (2014/12755-9). APB holds a MSc fellowship from CAPES. NCRO holds a PhD fellowship from CAPES.