Merkel cell carcinoma (MCC) is a primary neuroendocrine tumor of the skin1. This is a rare but highly aggressive tumor, characterized by rapid growth and tendency to nodal and vascular invasion, locoregional recurrence, and metastasization2. However, pleural involvement is anecdotal and only three cases have been described in the literature3–5. To reach a diagnosis, a high clinical suspicion and immunohistochemical study of histological specimens are essential3,4, given that it is a small cell neuroendocrine tumor that could be confused with other entities.
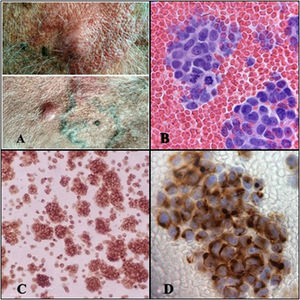
We report the case of a 69-year-old man who was admitted to our department with dyspnea and moderate pleural effusion. He was receiving immunosuppressive treatment after a kidney transplantation more than 10 years previously. One year before admission, an 8 mm papule was resected from the right side of the patient’s forehead, diagnosed histologically as Merkel cell carcinoma. Subsequently, when the patient presented relapse in the right cervical lymph node, an extended resection was performed with node dissection followed by adjuvant radiation therapy. Extension studies conducted at the time of diagnosis and during follow-up showed no distant lesions. On admission, he had numerous raised, hypervascularized skin lesions measuring up to 2.5 cm, predominantly on his trunk (Fig. 1A). Thoracentesis yielded a very bloody fluid, with pH 6.93, LDH 3279 U/l, glucose <30 mg/dl, and CRP 9.5 mg/l. The cell count showed only red blood cells and atypical cell aggregates suspected of malignancy and no other common pleural fluid components were identified. A monomorphic population of small, round, blue cells with little cytoplasm and hyperchromatic nucleus with nuclear chromatin in salt and pepper were observed on histology (Fig. 1B). The immunohistochemical study showed synaptophysin and cytokeratin 20 (CK20) expression with a dot-like pattern in 100% of neoplastic cells, specific to MCC (Fig. 1C, D). Computed tomography showed a large anterior mediastinal mass with pleural and pericardial involvement, large retroperitoneal and mesenteric lymph node clusters, and bilateral perirenal implants.
A) Raised skin lesions on the anterior aspect of the trunk. B) Cytological study of pleural fluid showing a monomorphic cell population of small, round, blue cells with scant cytoplasm and chromatin in salt and pepper, with numerous cells in mitosis (H-E cell block, ⋅40). C) Immunohistochemical study with synaptophysin expression (neuroendocrine marker), showing fine dot-like granular paranuclear expression (SYN, ⋅20). D) Immunohistochemical study showing positive cytokeratin 20 with dot-like perinuclear staining pattern in all neoplastic cells, specific to Merkel cell carcinoma (CK-20, ⋅40).
MCC was first described in 1972 when Toker published a series of 5 elderly patients with skin tumors and extensive lymphatic involvement. The author highlighted the formation of trabeculae or cords with scant tumor cytoplasm as the main characteristic1. Electrodense granules and positivity to neuroendocrine and epithelial staining were subsequently detected on electron microscopy and immunohistochemistry, characteristics that are shared with Merkel cells of the skin, so the entity became known as MCC3.
This is a rare tumor but one of the most aggressive, since up to one third of patients die as a result of the disease6. MCC usually affects elderly white patients, but has also been associated with immunosuppressive states, such as HIV infection, lymphoproliferative diseases, and solid organ transplantation7. Although it appears predominantly in photo-exposed areas, such as the head and neck, recent studies establish a relationship with Merkel polyomavirus in tumor carcinogenesis8. Incidence has increased in recent years, probably due to greater clinical knowledge, more precise methods for pathological diagnosis, aging of the population, and sun exposure9. In spite of its aggressiveness and its tendency to locoregional invasion and metastasization7,10, only 3 cases with pleural involvement have been published in the literature3–5.
The first, described by Watson in 1985, was a patient who, 20 years after resection of a skin tumor on his left hip, developed metastases and right pleural effusion. In this case, the diagnosis was only established from the cytological study of pleural fluid, since it resembled the histological samples obtained in the autopsy3. Years later, Payne et al.4 published the case of a 77-year-old woman who had pleural effusion 1 year after resection of an MCC skin lesion. On this occasion, the authors only described the macroscopic characteristics of pleural fluid as bloody, and arrived at their diagnosis after cytological and immunohistochemical study of the cell block. The third case presented bilateral pleural effusion 2 years after resection of the primary skin lesion. In this case, histopathological and immunohistochemical studies of pleural biopsy led to diagnosis5.
One novel aspect of our case is the description, for the first time in the literature, of the biochemical characteristics of the pleural fluid that demonstrate the enormous aggressiveness of the tumor. Decreased pH and glucose values in the pleura have been associated with a worse prognosis and more extensive pleural lesions11. In our case, these values were significantly reduced given the aggressiveness of the tumor and the extensive pleural involvement. This made it impossible to identify other common cellular components of pleural fluid, such as leukocytes or mesothelial cells, because they had been completely replaced by neoplastic cells.
In contrast to the cases published so far, our patient had been immunosuppressed for years since receiving a kidney transplant, so while both immunosuppressive treatment and solid organ transplantation have been described as risk factors for the development of this type of tumor7, this immunosuppression could also have enhanced the enormous aggressiveness and rapid progress of the tumor.
Pleural MCC represents a diagnostic challenge due in part to its rarity and the fact that it is a tumor that can be confused with metastases from other tumors such as lymphoma, small cell carcinoma, melanoma, Ewing sarcoma, and neuroblastoma. In these cases, therefore, it is essential to maintain a high level of suspicion that so that the immunohistochemistry techniques necessary to establish its diagnosis can be performed. This tumor, due to its neuroendocrine origin, often expresses markers, such as CD56, chromogranin, or synaptophysin. For diagnosis, however, positivity for cytokeratin 20 with a perinuclear pattern is a highly sensitive and specific marker12.
We believe that MCC may be a more frequent cause of malignant pleural effusion than currently recognized, and it may be responsible for pleural involvement even without evidence of skin lesions, given the descriptions published in the literature of cases of MCC with retroperitoneal or mediastinal involvement in which the primary tumor was not evident13,14. This highly aggressive and widely metastatic tumor may therefore not be recognized in the pleura unless the right immunohistochemical study to achieve a definitive diagnosis is conducted.
In conclusion, MCC is an entity that should be included in the differential diagnosis of malignant pleural effusion, especially in the case of small cell neuroendocrine tumors, even in the absence of skin lesions. In these cases, the determination of appropriate immunohistochemical markers will facilitate a definitive diagnosis.
Conflict of interestsThe authors state that they have no conflict of interests.
Please cite this article as: Soler-Sempere MJ, Alvárez-Fernández MO, Padilla-Navas I, Cabezas-Macián M, Sánchez-Hernández JF, García-Pachón E. Derrame pleural por carcinoma de células de Merkel. Arch Bronconeumol. 2021;57:715–717.