Long-term survival of lung transplantation (LT) patients is mainly limited by the development of chronic lung allograft dysfunction (CLAD). Lung retransplantation (LR) is an alternative for a selected population. The aim of this study was to review the LR experience in our center.
Patients and methodsWe conducted a retrospective study of patients undergoing LR between August 1990 and July 2017.
ResultsFourteen LR out of a total of 998 (1.4%) LT were performed. Twelve patients (85.7%) underwent LR due to CLAD: 10 (71.4%) because of bronchiolitis obliterans syndrome and 2 (14.3%) due to restrictive allograft syndrome. LR was performed in 2 patients within 30 days of the first LT. In those who underwent LR due to CLAD, mean time between the first LT and LR was 48 months, and mean duration of invasive mechanical ventilation was 32 days. The increase in FEV1 after LR was 24±18%. The best spirometry values were observed after 7.3 months. Mean survival of the cohort was 43.8 months. In patients with bronchiolitis obliterans syndrome, mean survival was 63.4 months, while in those with restrictive allograft syndrome, it was 19.5 months. Only 1 of the 2 early LR patients survived.
ConclusionLR is a therapeutic option in selected patients with CLAD, with acceptable survival. Indication for LR early after LT shows poor outcomes.
La supervivencia del trasplante pulmonar (TP) viene condicionada fundamentalmente por el desarrollo de disfunción crónica del injerto (DCI). El retrasplante pulmonar (RP) es una alternativa para una población seleccionada con DCI. El objetivo del estudio fue revisar la experiencia de RP en nuestro centro.
Pacientes y métodosSe ha realizado un estudio retrospectivo de los pacientes sometidos a RP entre agosto de 1990 y julio de 2017.
ResultadosSe realizaron 14 RP de un total de 998 (1,4%) TP. Doce RP se dieron por causa de DCI: 10 (71,4%) por síndrome de bronquiolitis obliterante y 2 (14,3%) por síndrome restrictivo del injerto. En 2 pacientes el RP se realizó en los 30 días siguientes al primer TP. En el RP por DCI el tiempo medio entre el TP y el RP fue de 48 meses. Tras el RP el tiempo medio de ventilación mecánica fue de 32 días. El incremento del FEV1 tras el RP fue del 24±18%. Los mejores valores en la espirometría se observaron a los 7,3 meses. La supervivencia media de la serie fue de 43,8 meses, en los pacientes con síndrome de bronquiolitis obliterante fue de 63,4 meses mientras que en los pacientes con síndrome restrictivo del injerto fue de 19,5 meses. Solo un paciente de los 2 RP precoces sobrevivió a este.
ConclusiónEl RP es una opción terapéutica en pacientes seleccionados con DCI. Sin embargo, estos resultados no son reproducibles si el RP se realiza en los primeros días.
Lung transplantation (LT) is the treatment of choice in selected patients with irreversible respiratory disease in whom medical treatment has not been effective. For the last 20 years, LT has been considered the best treatment for extending life expectancy and improving quality of life and exercise capacity in these patients.1 Despite the progress made, survival continues to be lower among lung transplant recipients than among recipients of other solid organs.2 According to data from the registry of the International Society for Heart and Lung Transplantation (ISHLT), median survival between January 1990 and June 2012 was 5.7 years.3 LT has particular characteristics that affect survival and make it different to other solid organ transplants. These include, most importantly, the constant exposure of the lung to the external environment, leaving it open to infections and the effects of pollution,4 microaspirations, gastroesophageal reflux, and the abundance of lymphoid tissue.5 All these elements are considered risk factors for the development of chronic lung allograft dysfunction (CLAD), which is the root cause of graft loss in the long term.
CLAD is understood as the progressive deterioration of lung function after transplantation caused by an intrinsic pulmonary process.6 One of the most striking forms of CLAD is bronchiolitis obliterans syndrome (BOS), defined by persistent and chronic obstruction with a loss of more than 20% of FEV1, that occurs in 75% of patients who develop progressive CLAD. In 2011, Sato et al. described a new phenotype that was called restrictive allograft syndrome (RAS).7,8 RAS is characterized by a functional loss of FEV1 of at least 20% compared to the best FVC or a loss >10% of TLC. These patients also have radiological findings in the form of pulmonary infiltrates that cannot be explained by other causes, and histological changes such as diffuse alveolar damage or fibrosis. Both phenotypes coexist in a considerable proportion of patients, but these mixed cases are not well defined and are usually classified as RAS.
The development of one phenotype or another has particular clinical relevance given that life expectancy after diagnosis differs between the two: patients with RAS have a more rapid clinical course, and a median survival after diagnosis of only 1.5 years, compared to the 4 years of patients who are diagnosed with BOS.9 In general, the medical treatment of these syndromes has been unsuccessful, so the growing interest in offering the patient a second chance with a second transplant is no surprise. In fact, lung retransplantation (LR) in selected patients is the only treatment that has been shown to have an impact on survival.10–12 According to data from the ISHLT, between January 1995 and June 2013, 5.1% of unilateral transplants and 3.4% of bilateral transplants were retransplantations, the most frequent indication being BOS.3 In this study, we review a series of patients who have undergone this procedure since the inception of the LR program in our center.
Patients and MethodsWe retrospectively reviewed the data of patients who underwent LR in our center between August 1, 1990 and July 31, 2017. The most common diseases were chronic obstructive pulmonary disease, interstitial lung disease, and bronchiectasis/cystic fibrosis. Patients who met the criteria set out in the LR candidate selection consensus were considered potential candidates.13
The possibility of LR is routinely considered in patients who progress poorly after LT. In all cases, a consensus decision is taken by the hospital LT committee. When LR is indicated, factors such as potential surgical difficulties, microbiological isolates, the general status of the patient, fragility, and the role of the other organs are taken into account.
This study is a retrospective chart review of the outcomes of all lung retransplantations carried out since the inception of the LR program. The following variables were analyzed: LR indication, immunosuppressive treatment, spirometric progress, length of stay in the intensive care unit (ICU) and in the hospital, time on mechanical ventilation, microbiological and immunological complications, mortality and its causes.
For the descriptive study, quantitative variables are presented as mean, standard deviation, and range. Qualitative variables are shown as frequencies and percentages. The Kaplan–Meier method was used for the survival calculations.
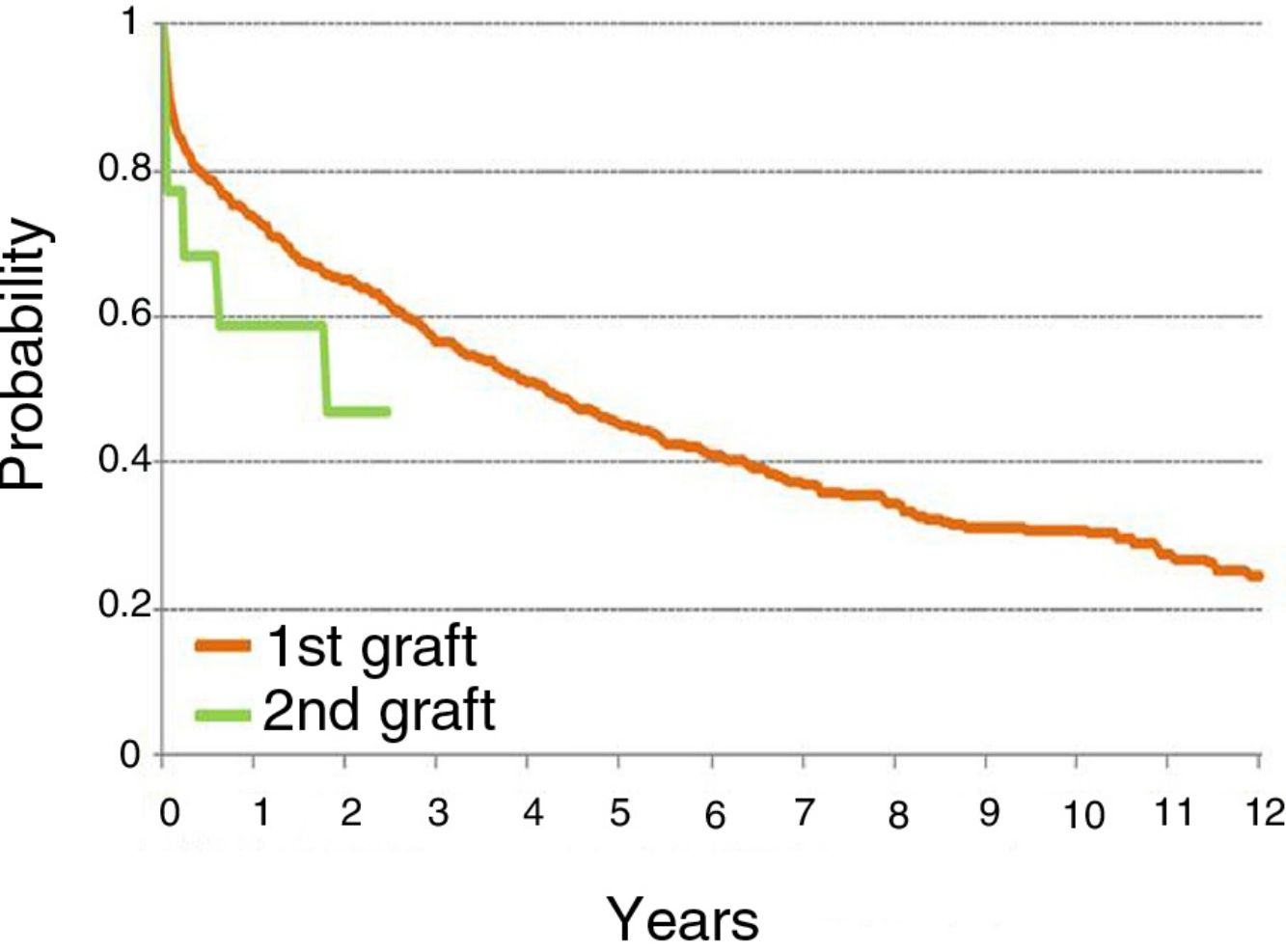
ResultsFrom August 1990 to July 2017, 14 LR were performed, accounting for 1.4% of the total 998 LTs performed in that period. Of these, 10 (71.4%) patients were women and 4 (28.6%) were men. Mean age at the time of LR was 31.7 years (range 17.6–49.5). Clinical characteristics and complications of the study population after the first transplant and the LR are shown in Table 1.
Complications Among LR Patients.
| S | Initial Diagnosis | LT | Reason for LR | LR | Comorbidities pre-LR | Age at LR | Days of UCI Stay | Time on IMV | CLAD LR | Date Death | Cause of Death | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | RF | |||||||||||
| F | IPF | 1990 LSLT | CLAD | 1993 RSLT | No | No | 23 | – | – | No | 1993 | Surgical complications |
| F | CF | 1997 DL | CLAD | 2017 DL | Yes | Yes | 42 | 10 | 4 | No | No | |
| M | COPD | 1998 LSLT | Surgical complication | 1998 LSLT | No | No | 49 | 37 | 33 | No | 1998 | Multiorgan failure |
| F | CF | 1999 DL | CLAD | 2001 LSLT | No | No | 17 | 7 | 7 | No | 2001 | Hemorrhagic shock |
| F | IPF | 2005 DL | CLAD | 2011 RSLT | No | No | 15 | 9 | 7 | BOS | 2017 | Massive hemoptysis |
| M | CF | 2006 DL | CLAD | 2009 RSLT | Yes | Yes | 31 | 89 | 86 | No | 2011 | Pulmonary aspergillosis |
| F | HX | 2007 DL | CLAD | 2010 RSLT | No | No | 32 | 41 | 36 | BOS | No | |
| M | CF | 2009 DL | CLAD | 2013 DL | No | No | 18 | 36 | 34 | No | No | |
| F | CF | 2009 DL | CLAD | 2016 LSLT | Yes | Yes | 33 | 20 | 12 | No | No | |
| M | CF | 2010 DL | CLAD | 2014 DL | No | No | 19 | 47 | 36 | No | No | |
| F | HP | 2012 LSLT | Surgical complication | 2012 LSLT | No | No | 57 | 24 | 17 | BOS | No | |
| F | NSIP | 2013 DL | CLAD | 2015 RSLT | Yes | Yes | 51 | 37 | 31 | RAS | 2016 | Pneumonia |
| F | BO | 2013 DL | CLAD | 2015 DL | No | No | 3 | 94 | 94 | No | 2015 | Angioinvasive pulmonary aspergillosis |
| F | COPD | 2013 DL | CLAD | 2016 DL | No | No | 54 | 33 | 22 | No | No | |
BO: bronchiolitis obliterans; CF: cystic fibrosis; CLAD: chronic lung allograft dysfunction; COPD: chronic obstructive pulmonary disease; DL: double-lung; DM, diabetes mellitus; Dx: diagnosis; F: female; HP: hypersensitivity pneumonitis; HX: histiocytosis X; ICU, intensive care unit; IMV: invasive mechanical ventilation; IPF: idiopathic pulmonary fibrosis; LR: lung retransplantation; LSLT: left single-lung transplant; LT: lung transplantation; M: male; RAS: restrictive allograft syndrome; RF: renal failure; RSLT: right single-lung transplant; S: sex.
LR was indicated in 12 (85.7%) patients due to CLAD; 10 (71.4%) in the form of BOS and 2 (14.3%) in the form of RAS. In the 2 remaining cases, LR was indicated due to surgical complications occurring in the immediate postoperative period.
In the 12 patients with CLAD, mean lung function at the time of LR was FVC 49.6%±14.2% and FEV1 34.8%±11.7%. Mean time between the first LT and LR was 48±58.2 months. We performed 5 (35.7%) bilateral LRs, 4 (28.6%) left unilateral LRs, and 5 (35.7%) right LRs.
The initial immunosuppression protocol consisted of a calcineurin inhibitor (tacrolimus), a purine synthesis inhibitor, principally mycophenolate mofetil, although azathioprine was used in one case, and all patients received methylprednisolone. Five (35.7%) patients received basiliximab: 2 were pediatric LT recipients, in whom basiliximab was used for induction therapy, while 3 were adults who received it the immediate LT postoperative period due to renal failure (RF) which ruled out the use of anticalcineurinics.
Mean hospital stay after LR was 48±29 days, mean ICU stay was 37±28 days, and mean time on invasive mechanical ventilation was 32±29 days.
Best functionality was achieved 7.3±9.9 months post-LR, with an increase in FEV1 after LR of 24%±18% and FVC of 59.4%±15.8% from a mean baseline FEV1 of 58%±17.4%.
Perioperative ComplicationsThe perioperative mortality rate was 21.4% (3 patients). One patient died at 24h due to surgical complications. Another died at 8 days, due to a ruptured pulmonary artery at the anastomosis site, causing hemorrhagic shock, and the third could not be weaned from extracorporeal circulation and required extracorporeal membrane oxygenation. She died at 18 days due to recurrent bleeding that even required left pneumonectomy. Angioinvasive pulmonary aspergillosis was found on autopsy.
Three (21.4%) patients presented primary graft dysfunction, 2 grade III and 1 grade I.
Late ComplicationsBetween day 30 post-transplant and completion of the study, mortality was 28.6% (4 patients). The main causes were infectious diseases in 3 cases and massive hemoptysis in 1. During this period, 3 (21.4%) patients developed fungal infections: 2 (14.3%) had tracheobronchitis caused by Aspergillus fumigatus and 1 (7.1%) cavitary pulmonary aspergillosis caused by Aspergillus niger. Two (14.3%) patients presented cytomegalovirus replication and required treatment with valganciclovir. Five (35.7%) patients presented colonization by multidrug resistant bacteria: 4 (28.6%) Pseudomonas aeruginosa and 1 (7.1%) Burkholderia multivorans. With regard to bronchial sutures, only one patient presented 15% stenosis of the intermediate bronchus which required no specific action.
Acute and Chronic Cellular RejectionThe incidence of biopsy-confirmed acute cellular rejection was 14.3%, and involved 2 patients, one with stage A1 and the other stage A2. Both patients recovered and only the one with the higher grade needed treatment.
CLAD was diagnosed in 4 (28.6%) patients: 3 were BOS and 1 was RAS. Mean time from LR to diagnosis of CLAD was 30±19.6 months, and mean lung function at the time of diagnosis was FVC 37.1%±15.5% and FEV1 28.3%±9.3%. MTOR inhibitors were used as second-line treatment and were indicated to replace mycophenolate mofetil in 3 (21.4%) patients due to recurrence of CLAD, 2 (14.3%) of whom received rapamycin and one (7.1%) everolimus.
SurvivalMean survival after LR was 43.8±10.3 months with a median of 74±36.9 months. If the 3 (21.4%) patients who died in the postoperative period are excluded, median survival was 55.8±10.6 months with a median of 74±39.5 months.
Mean survival by type of CLAD was 63.4±12.4 months with a median of 74±40.5 months in patients with BOS, while patients with RAS had a mean survival of 19.5±9.5 months with a median of 6 months.
DiscussionCLAD remains the main limitation to survival after LR. It occurs in 50% of patients within 5 years after the procedure, and accounts for about 30% of deaths between 3 and 5 years after the LT.3,7 To date, no specific treatment exists for CLAD. However, LR in selected patients can lengthen survival. The few studies published on this issue are case series, probably due to changes made in the criteria and indications for LT over the years. For example, the consensus published in 1998 did not consider the option of the LR14; it was first mentioned in the consensus of 2006,15 but it was not discussed specifically until 2015. The aim of this study was to analyze the outcome of LR in terms of survival in our setting.
According to ISHLT data, the mean survival of LRs performed between January 1990 and June 2012 was 2.5 years. The mean survival obtained in our series was 3.7 years, a figure clearly lower than that obtained after a first LT. Either way, the outcome of a second LT is always poorer than that of the first LT. Patients are selected for LR using the same ISHLT criteria as for a first LT. Furthermore, decisions are taken by consensus among the entire multidisciplinary team that seeks to maintain the principle of fair use and equal access for all patients.
A fact to bear in mind is that survival after LR is higher in patients who undergo the procedure more than 1 year after their initial LT. Another important fact revealed by this study is that survival after LR is longer if it is performed for BOS rather than RAS. When we analyze LR patients who survived more than 3 months after the procedure, median survival was 63 months in patients who were retransplanted due to BOS compared to 20 months who underwent the procedure due to RAS. This is consistent with previously published data.16 The Louvain group published a descriptive study in which they found a survival of 1.7 years in patients undergoing LR due to RAS, compared to 5.1 years in patients with BOS. Other authors have analyzed survival after LR, such as Thomas et al.,17 who described a mean survival of 2.6 years or Novick et al.,18 who reported a mean survival after LR was 2.5 years. However, both studies were performed after the RAS phenotype was described, and it is likely that both phenotypes had not been well defined at the time of publication. Given the scarcity of published studies, the risk factors that explain the differences in terms of survival between the two phenotypes are not well understood. It could be speculated that the reason for worse survival in patients with RAS is the presence of a fibrotic component, causing pleural adhesion that makes the surgical procedure more complicated. Moreover, the increased need for extracorporeal circulation in this subgroup of patients, along with increased perioperative mortality due to bleeding and an increase in the number of re-interventions, has an impact on graft survival in the long term.
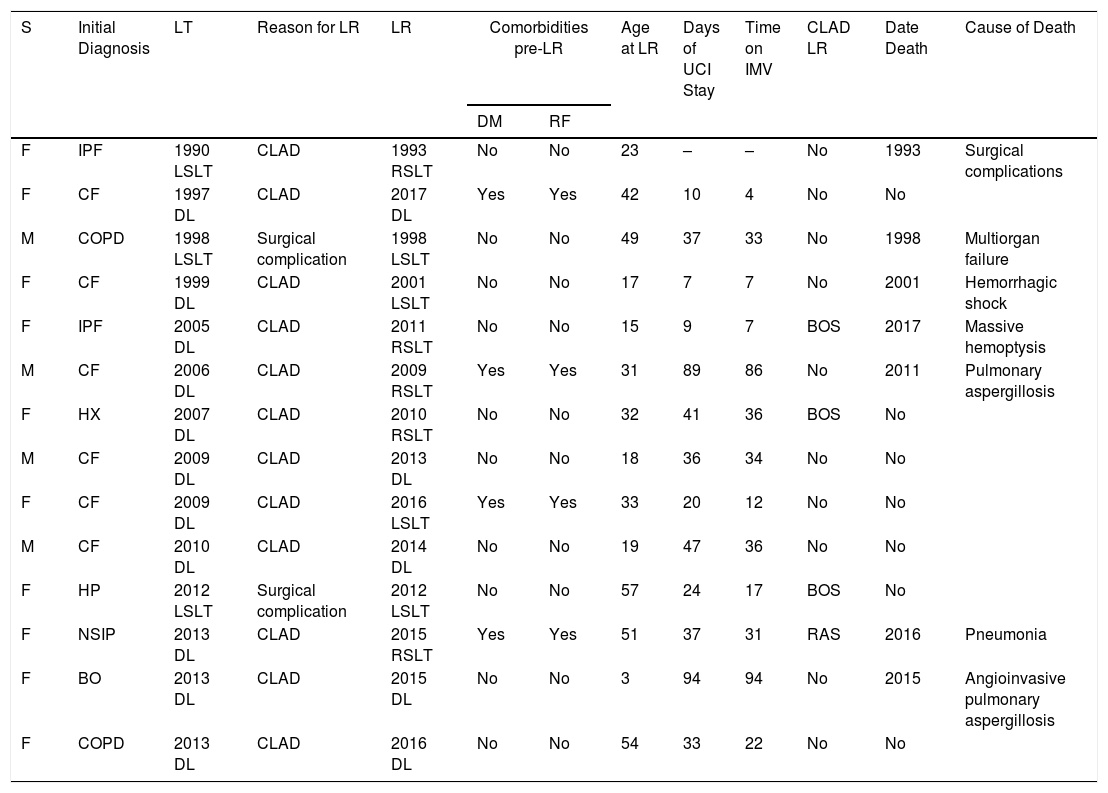
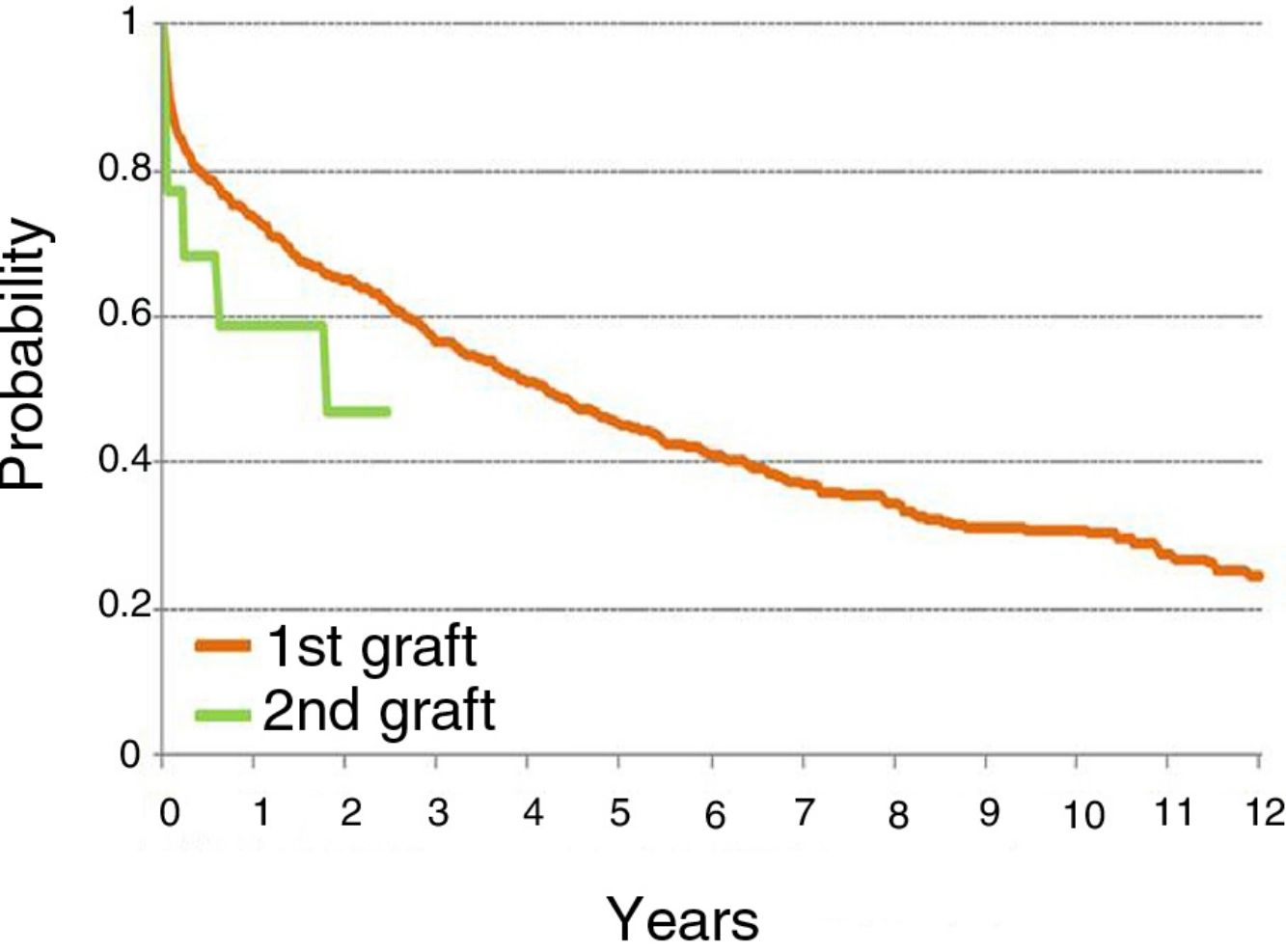
In our series, survival after the first LT is better than after LR, with an overall survival rate at 1 year after the first LT of 73% compared to 58% in LR patients (see summary in figure). Given the small number of patients, a subgroup analysis differentiating single LRs from double LRs was not possible; the greater mortality observed in single LRs may be explained by removal and implantation difficulties due to adhesions, and also because these patients were older than those undergoing double LR.
Another point of interest in this study is that patients who survived the postoperative period ultimately survived longer than any of the patients who were treated with the alternative strategies presently available for the treatment of graft dysfunction, and showed a longer survival after diagnosis of CLAD than expected.
The causes of perioperative mortality described in our series include those related to the surgery itself. These data are consistent with those published by the ISHLT, in which the main causes of perioperative mortality include primary graft dysfunction, infections not associated with cytomegalovirus (CMV), surgical complications, and cardiovascular causes. The latter factors were not observed in our series, due to the young age of our population.
Mean ICU stay and time on IMV are higher (37 and 32 days respectively) in our LR series than in other series of first LTs, for example, that of Riera et al., also carried out in our hospital.19 The authors analyzed 100 patients transplanted between September 2011 and May 2013 with a mean ICU stay and time on IMV of 21 and 15 days respectively.
The most common comorbidities in our series were diabetes mellitus (DM) and RF. In 6 (42.9%) patients who underwent LR, DM was the most frequent previous comorbidity. Four (28.6%) patients had RF, defined as a glomerular filtration rate less than 60ml/min/m2. These data are consistent with previously published data. The Louvain group found that 23% of their series had RF and 51% had DM. These data were similar when compared with patients with RAS (22% with RF and 34% with DM).
The most common long-term complications recorded in our patients were infectious. Five (36%) patients were colonized by multidrug resistant bacteria, 2 had fungal infections, and 2 patients developed cytomegalovirus replication that required treatment.
Immunological problems were the second most common complications in our series. Three (21.4%) patients had acute cellular rejection that was treated by adjusting steroid doses, and 4 (28.6%) patients developed CLAD again, 1 of which occurred within a year of the RL. These data are consistent with those described in the ISHLT where it is observed that 53.4% of retransplanted patients will develop BOS within 5 years. One could hypothesize that this is a population with greater immunological predisposition to acute cellular rejection. For this reason, more studies are needed to determine all the potential risk factors, so that they can be avoided.
As in a first LT, probably one of the factors that has most impact on survival outcomes after LR is the status of the recipient. In this respect, Novick et al.18 describe better 1-year survival in ambulatory patients compared to hospitalized patients and those receiving ventilatory support. Therefore, as with a first LT, the outcomes of LR are better when it is performed electively.
This study has limitations due to its retrospective, single-center design. However, it is the first to describe the experience in a busy transplantation center in our setting. Further studies based on descriptions of patient series and clinical experience are needed to establish the efficacy of LR in a population likely to benefit from the intervention, and to define the characteristics of a selected population that will guarantee good outcomes in terms of survival and good graft function. Another factor to bear in mind, despite the sample size, is that patients undergoing LR due to RAS have poorer outcomes. However, the absence of a specific treatment for CLAD means that LR is currently the only real alternative for a selected population that may have an impact on survival. It is clear that more studies have to be carried out in order to determine the reasons for this situation.
Conflict of InterestsThe authors state that they have no conflict of interests.
Please cite this article as: Revilla-López E, Berastegui C, Sáez-Giménez B, Lopez-Meseguer M, Monforte V, Bravo C, et al. Resultados del retrasplante pulmonar por disfunción crónica del injerto pulmonar en un centro trasplantador: Hospital Vall D’Hebron de Barcelona. Arch Bronconeumol. 2019;55:134–138.