Since the emergence of the 2019 novel coronavirus (SARS-CoV-2) infection in Wuhan, China in December 2019, it has rapidly spread across China and many other countries. Although the majority of patients have only mild symptoms, some of them deteriorate and develop respiratory failure owing to severe acute respiratory distress syndrome (ARDS) requiring ICU admission, intubation, and mechanical ventilation.1,2 For patients needing prolonged mechanical ventilation, percutaneous or surgical tracheostomy have been proposed,3–5 however these patients are susceptible to developing increased nosocomial infections, mortality rates and existence of relevant impacts on their quality of life in the months following Intensive Care Unit (ICU) discharge. The aim of present study was to describe patient characteristics, hospital course, and long-term outcomes (at six months) such as mortality, quality of life, functional status, and persistent symptoms of critical COVID-19 patients who needed a tracheostomy during the March–April 2020 outbreak.
In this prospective, multicenter, observational cohort study, we included all patients admitted to ICU with severe respiratory failure by COVID-19 requiring tracheostomy for prolonged mechanical ventilation in seven Hospitals in Northwestern Spain, during the March–April 2020 outbreak. A confirmed case of COVID-19 was defined by a positive result on a reverse-transcriptase-polymerase-chain-reaction (RT-PCR). The following information was collected during ICU admission: demographics, coexisting disorders, treatments, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, laboratory tests, nosocomial infections, complications during tracheostomy, duration of mechanical ventilation, time to tracheostomy after mechanical ventilation was initiated, time to decannulation after tracheostomy was performed, ICU and Hospital outcomes. All patients who survived ICU admission were included to assess health-related quality of life (HRQOL), functional status, and persistent symptoms, using a structured telephone survey conducted by designated trained research coordinators at participating sites, six months after hospitalization. Patients were also asked to retrospectively recount their quality of life and functional status 3 months before COVID-19. HRQOL was assessed using the EuroQol Group Association five-domain, three level questionnaire (EQ-5D-3L), which consists in two sections: the descriptive system and the visual analogue scale. The descriptive system measures five domains of health including mobility, self-care, usual activities, pain/discomfort, and anxiety/depression and assesses each domain across three levels: no problems, some problems, or extreme problems. The visual analogue scale (EQ-VAS) represents 0=worst imaginable health and 100=best imaginable health (5). Functional status was assessed according to the recently described post-COVID-19 functional status scale (PCFS), which consists in an ordinal scale for assessment of patient-relevant functional limitations6 (0: No limitations in my everyday life, 1: Negligible limitations with persistent symptoms, 2: Limitations in my everyday life, and occasionally need avoid or reduce usual activities, 3: Limitations in my everyday life, and I am not able to perform all usual activities, 4: Severe limitations. I am dependent from another person due to symptoms). Other persistent symptoms potentially correlated with COVID-19 were obtained also. All analyses were performed using R (version 4.0.2; R Foundation for Statistical Computing) and IBM SPSS (version 26; SPSS, Inc, Chicago, IL, USA). The ethics committee of Galicia, Spain (code No. 2020-188) approved this study. Informed consent was obtained from all participants.
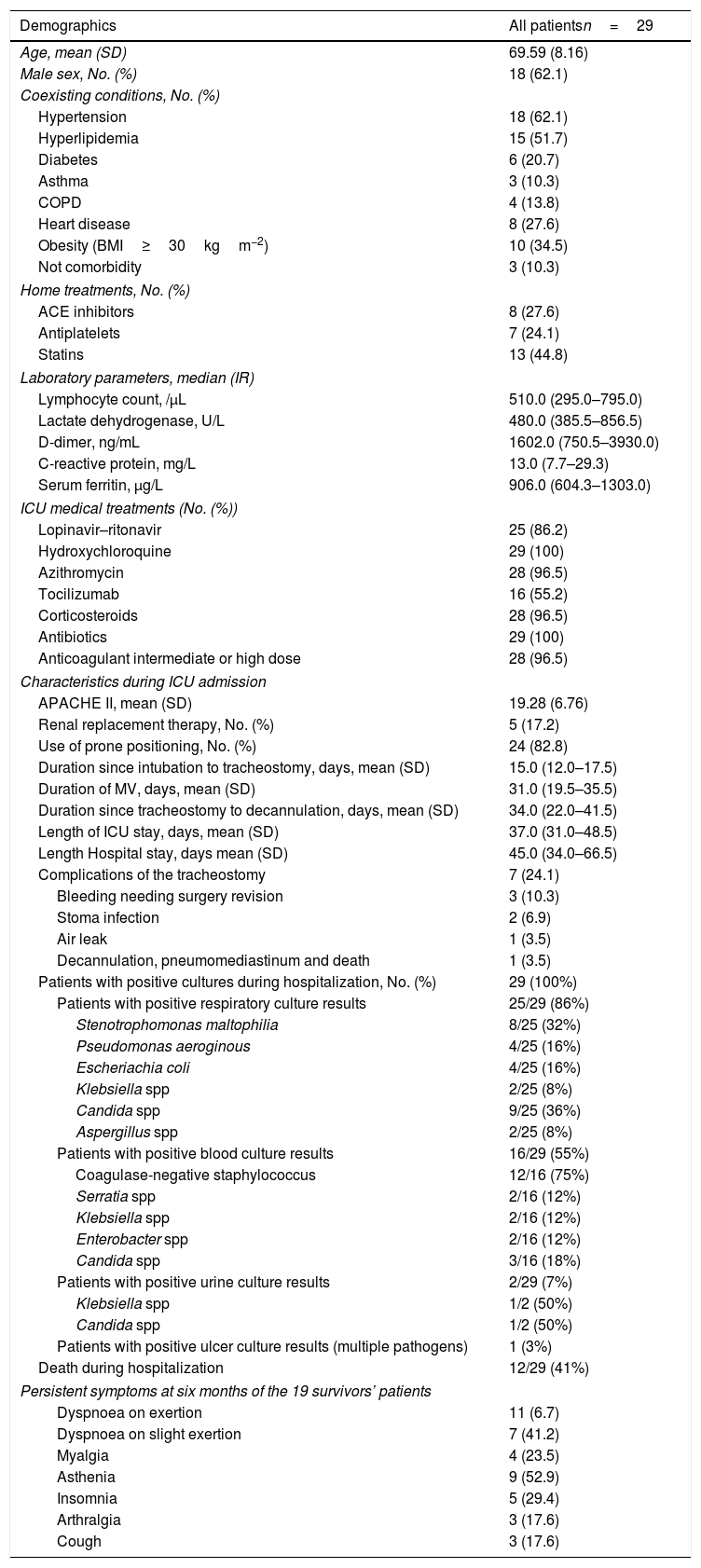
From March 1 to April 31, 2020, a total of 98 COVID-19 patients were intubated needing mechanical ventilation in the ICUS in seven Hospitals of Galicia. Of these patients, twenty-nine (29.6%) needed tracheostomy and they were included in the present study. Mortality rate during Hospital admission was higher in patients who needed tracheostomy (12/29 (41%)) compared with patients who did not need tracheostomy (13/69 (18%), (p<0.001). Of the 29 tracheostomized patients, 17 (59%) remain alive at six months after ICU discharge. Table 1 displays patient characteristics, clinical course, tracheostomy complications, nosocomial infections, treatments, and ICU and Hospital outcomes of the 29 patients. At six months the 17 survivors responded to a 6-month follow-up survey. Of the survivors, worsened quality of life measured with the EQ-VAS was observed among 84% of patient. The EQ-VAS decreased from 87.06 (14.48) to 61.18 (18.33) (p<0.001). With the EQ-5D, we observed that 76% patients had moderate to extreme problems in any of the five dimensions studied. Eleven (65%) patients reported problems with mobility, 11 (65%) patients reported problems with usual activities, 8 (47%) patients reported problems with self-care activities, 9 (52%) patients reported pain or discomfort, and 10 (59%) reported anxiety or depression. At six months interview, fourteen (82%) and 10 (59%) patients had lowered one and two grades respectively in the Post COVID-19 functional status scale. Eleven (65%) patients had persistent functional limitations (grade 2–4 in the PCFS). A high proportion of patients recounted dyspnoea on exertion (65%), asthenia (53%), insomnia (29%) myalgia (23%), and arthralgia (18%). Only 2 (12%) patients were completely free of persistent symptoms at six months (Table 1).
Demographics and clinical characteristics during ICU and Hospital admission of the study sample (n=29).
| Demographics | All patientsn=29 |
|---|---|
| Age, mean (SD) | 69.59 (8.16) |
| Male sex, No. (%) | 18 (62.1) |
| Coexisting conditions, No. (%) | |
| Hypertension | 18 (62.1) |
| Hyperlipidemia | 15 (51.7) |
| Diabetes | 6 (20.7) |
| Asthma | 3 (10.3) |
| COPD | 4 (13.8) |
| Heart disease | 8 (27.6) |
| Obesity (BMI≥30kgm−2) | 10 (34.5) |
| Not comorbidity | 3 (10.3) |
| Home treatments, No. (%) | |
| ACE inhibitors | 8 (27.6) |
| Antiplatelets | 7 (24.1) |
| Statins | 13 (44.8) |
| Laboratory parameters, median (IR) | |
| Lymphocyte count, /μL | 510.0 (295.0–795.0) |
| Lactate dehydrogenase, U/L | 480.0 (385.5–856.5) |
| D-dimer, ng/mL | 1602.0 (750.5–3930.0) |
| C-reactive protein, mg/L | 13.0 (7.7–29.3) |
| Serum ferritin, μg/L | 906.0 (604.3–1303.0) |
| ICU medical treatments (No. (%)) | |
| Lopinavir–ritonavir | 25 (86.2) |
| Hydroxychloroquine | 29 (100) |
| Azithromycin | 28 (96.5) |
| Tocilizumab | 16 (55.2) |
| Corticosteroids | 28 (96.5) |
| Antibiotics | 29 (100) |
| Anticoagulant intermediate or high dose | 28 (96.5) |
| Characteristics during ICU admission | |
| APACHE II, mean (SD) | 19.28 (6.76) |
| Renal replacement therapy, No. (%) | 5 (17.2) |
| Use of prone positioning, No. (%) | 24 (82.8) |
| Duration since intubation to tracheostomy, days, mean (SD) | 15.0 (12.0–17.5) |
| Duration of MV, days, mean (SD) | 31.0 (19.5–35.5) |
| Duration since tracheostomy to decannulation, days, mean (SD) | 34.0 (22.0–41.5) |
| Length of ICU stay, days, mean (SD) | 37.0 (31.0–48.5) |
| Length Hospital stay, days mean (SD) | 45.0 (34.0–66.5) |
| Complications of the tracheostomy | 7 (24.1) |
| Bleeding needing surgery revision | 3 (10.3) |
| Stoma infection | 2 (6.9) |
| Air leak | 1 (3.5) |
| Decannulation, pneumomediastinum and death | 1 (3.5) |
| Patients with positive cultures during hospitalization, No. (%) | 29 (100%) |
| Patients with positive respiratory culture results | 25/29 (86%) |
| Stenotrophomonas maltophilia | 8/25 (32%) |
| Pseudomonas aeroginous | 4/25 (16%) |
| Escheriachia coli | 4/25 (16%) |
| Klebsiella spp | 2/25 (8%) |
| Candida spp | 9/25 (36%) |
| Aspergillus spp | 2/25 (8%) |
| Patients with positive blood culture results | 16/29 (55%) |
| Coagulase-negative staphylococcus | 12/16 (75%) |
| Serratia spp | 2/16 (12%) |
| Klebsiella spp | 2/16 (12%) |
| Enterobacter spp | 2/16 (12%) |
| Candida spp | 3/16 (18%) |
| Patients with positive urine culture results | 2/29 (7%) |
| Klebsiella spp | 1/2 (50%) |
| Candida spp | 1/2 (50%) |
| Patients with positive ulcer culture results (multiple pathogens) | 1 (3%) |
| Death during hospitalization | 12/29 (41%) |
| Persistent symptoms at six months of the 19 survivors’ patients | |
| Dyspnoea on exertion | 11 (6.7) |
| Dyspnoea on slight exertion | 7 (41.2) |
| Myalgia | 4 (23.5) |
| Asthenia | 9 (52.9) |
| Insomnia | 5 (29.4) |
| Arthralgia | 3 (17.6) |
| Cough | 3 (17.6) |
Date are number (percentage), median (interquartile range), or mean (standard deviation). COPD, chronic obstructive pulmonary disease; ACE: angiotensin-converting-enzyme inhibitors; APACHE II: Acute Physiology and Chronic Health Evaluation II; BMI: body mass index.
This study represents a description of critically ill COVID-19 patients that required a tracheostomy in seven hospital in the northwest of Spain. These patients had a high rate of mortality, nosocomial infections, and prolonged ICU and Hospital stay similar to other previous studies.3–5 At six months, a large proportion of survivors had persistent symptoms and reduced quality of life and functional status.
The limitation of this study includes the relatively small number of patients, and that the study was conducted in seven hospitals in Northwest Spain and may not be representative of other patient populations. However, these findings have implications for health service planning and for planning the ongoing support and treatment of survivors of critical illness by COVID-19. There is a need to identify strategies during the hospital course, such as early rehabilitation, or after hospital discharge, such as follow-up clinics, to improve long time quality of life for COVID-19 survivors.
Summary statementOur critically ill Covid-19 patients who needed a tracheostomy had a high rate of death, nosocomial infections, and prolonged ICU and Hospital stay. At six months, a large proportion of survivors had persistent symptoms and reduced quality of life and functional status.
SupportSupport was provided solely from institutional and departmental sources.
Authors’ contributionsConception of the study: Manuel Taboada.
Study design: Manuel Taboada.
Data collection: All authors.
Data analysis: Teresa Seoane-Pillado.
Drafting the manuscript: All authors helped to revise the draft of the manuscript.
Editing and approval of the manuscript: All authors.
FundingNo funding provided.
Conflicts of interestThe authors declare the absence of conflict of interests.
The authors are grateful to physicians and nurses of the participating Hospitals.