Cystic fibrosis related diabetes (CFRD) is a strong determinant for lung function decline and increased mortality. Insulin treatment of CFRD is reportedly beneficial for this situation. We report on the long-term impact of insulin treatment of CFRD on pulmonary function and nutritional status in a CF male patient since diagnosis of diabetes.
We report the case of a patient diagnosed with CF at the age of 16. Two years later, he experienced a rapidly evolving decrease in pulmonary function, some months later criteria were met warranting lung transplantation. Concomitantly, he was diagnosed with CFRD and insulin therapy was started. Lung function (spirometry), nutritional status (body mass index) and metabolic control (HbA1c) were determined every 3 months. After the introduction of insulin treatment, pulmonary function and nutritional status progressively improved and good glycemic control was achieved. The significant and sustained improvement in pulmonary function allowed for the patient's withdrawal from the lung transplantation program within 4 months, a situation which has been maintained until now, 8 years later.
The long follow-up of our patient documents the rapid and prolonged beneficial effect of proper metabolic control of CFRD on the respiratory deterioration in CF.
La diabetes relacionada con la fibrosis quística (DRFQ) es un factor determinante del empeoramiento de la función pulmonar y del incremento de mortalidad. El tratamiento con insulina mejora esta situación.
Se presenta el caso de un paciente diagnosticado de fibrosis quística (FQ) a los 16 años. Al cabo de dos años presentó un deterioro rápido de la función pulmonar, cumpliendo meses después criterios de trasplante pulmonar; concomitantemente, fue diagnosticado de DRFQ e inició insulinoterapia. En el seguimiento se evaluaron la función pulmonar por espirometría, el estado nutricional (índice de masa corporal) y el control metabólico (HbA1c) cada 3 meses. Tras iniciar insulinoterapia, obtuvo un buen control metabólico y mejoría progresiva espirométrica y del estado nutricional. A los 4 meses ya no cumplía criterios de trasplante pulmonar, situación que se mantiene a los 8 años.
El largo seguimiento documenta un efecto beneficioso rápido y prolongado del buen control metabólico de la DRFQ sobre el deterioro respiratorio en FQ.
Lung function alterations are the main factors responsible for the high mortality of patients with cystic fibrosis (CF). In recent decades, the specialized treatment of CF has significantly increased their life expectancy,1 and the current average survival is more than 30 years. Lung transplantation is the only therapy available in the final stages of this lung disease.2 With the increased survival in CF, more and more patients have the possibility of developing extra-pulmonary complications. CF-related diabetes (CFRD) is the most frequent comorbidity and constitutes at the same time a marker for poorer prognosis and greater mortality.3,4 The prevalence of CFRD increases with age3,5,6 and its appearance is usually preceded by moderate alterations in the metabolism of carbohydrates, while there are reports of worsened lung function and nutritional state years before its diagnosis.3,7–9 The treatment of CFRD with insulin seems to improve the respiratory and nutritional states of these patients.5,10,11 There are few studies about the duration of this beneficial effect. In patients with CF who require lung transplantation, the prevalence of CFRD is 28.6%.12 We present the beneficial and prolonged effect of insulin therapy on lung function and on nutritional state in a male with CF who started with insulin at the time of his diagnosis with CFRD, coinciding with the indication of lung transplantation.
Patient and MethodsClinical NotesA male patient was diagnosed with CF at the age of 16 due to repeated bronchopulmonary infections and two pathological sweat tests (95 and 86mequiv./l chloride ion). Since this age, he has presented chronic bronchopulmonary infection by Staphylococcus aureus sensitive to methicillin and Pseudomonas aeruginosa sensitive to ciprofloxacin, aminoglycosides, sodium colistimethate and the majority of antipseudomonal beta-lactams. High resolution computed axial tomography showed the presence of diffuse bronchiectasis, predominantly in the upper lobes. At the time of CF diagnosis, he had a forced vital capacity (FVC) of 4600ml (106%) and a forced expiratory volume in one second (FEV1) of 3600ml (101%). One year later, the patient was diagnosed with allergic bronchopulmonary aspergillosis (ABPA) and was treated with oral corticosteroids for 6 months together with maintenance azithromycin (500mg administered 3 times a week), with resolution of the ABPA flare-up. The third year after CF diagnosis, lung function and nutritional state worsened drastically. The patient presented numerous pulmonary exacerbations that required several cycles of oral and intravenous antibiotics, with a response that became poorer and poorer and the functional deterioration became indispensible. In the end, the patient required home oxygen therapy. In an attempt at controlling the lung inflammation and slowing down the respiratory deterioration, he also received moderate doses of oral corticosteroids (16mg/day of methylprednisolone). At the age of 19, he was sent to the lung transplantation unit at our center due to the rapidly progressing respiratory deterioration, with an FEV1 of 30% predicted value and the need for continuous oxygen therapy; at that time he was being treated with 20mg/48h of methylprednisolone (0.2mg/kg/day). As part of the initial transplantation study protocol, the hydrocarbonated metabolism was evaluated by means of oral glucose overload, which showed evidence of the existence of CFRD.13 He presented no cardinal symptoms nor did he have a family history of diabetes. Treatment was initiated with insulin at multiple doses after receiving diabetes education. Exocrine pancreatic function was always conserved, with no required substitutive therapy.
MethodsLung FunctionLung function was evaluated by forced spirometry every 3–4 months, expressing FVC and FEV1 as percentage of the predicted value for age, ethnicity, sex, weight and height. Nutritional state was estimated with body mass index [BMI=weight (kg)/height2 (m)] related with age and sex. The criteria for lung transplantation used were those defined by Prados et al.14 and Liou et al.15.
CFRD Study and ControlFor the diagnosis of the hydrocarbonated alteration, a fasting oral glucose overload study (administration of 75g of glucose) was performed, using the criteria of the American Diabetes Association.13 CFRD treatment was initiated at multiple doses with regular insulin (Actrapid®) before breakfast, lunch and dinner, and NPH insulin (Insulatard®) at lunch and dinner, adjusting the doses according to the capillary glycemia (before and 2h after meals and at nighttime) depending on the servings of carbohydrates ingested. For the evaluation of the CFRD control, glycated hemoglobin (HbA1c) was determined every 3 months (HPLC Menarini; normal value, 5.3%±0.3%).
Molecular StudyDNA analysis was done with the Elucigene™ CF29 v.2 method (Tepnel Diagnostics Ltd., Oxfordshire, Great Britain). The Delta 508F and R553X mutations were identified in compound heterozygosis, confirmed with direct sequencing of the products of the chain reaction of the polymerase of the exons corresponding with the CFTR (cystic fibrosis transmembrane conductance regulator) gene.
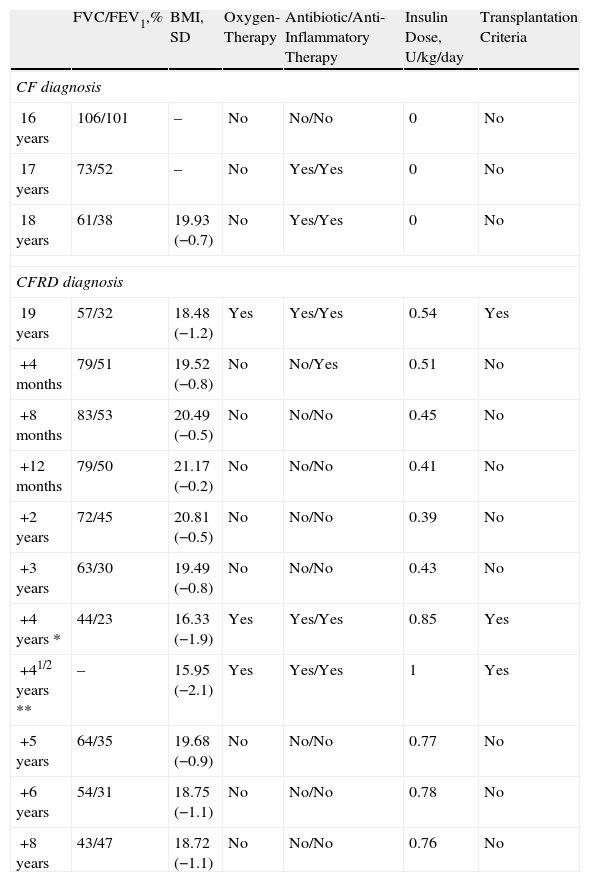
ResultsAfter the start of the treatment with insulin, a progressive improvement was observed in lung function and nutritional state (Table 1) and within 4 months the patient no longer met the criteria for lung transplantation. The doses of corticosteroids were gradually reduced, and five months after the start of insulin therapy, corticosteroid treatment was able to be suspended without the patient presenting any lung exacerbations during their withdrawal. Home oxygen therapy was also suspended and the spirometry parameters improved to the levels prior to the diagnosis of CFRD (Table 1). Ten months later, the patient was able to do regular exercise.
Evolution of the Lung Function and Nutritional State.
| FVC/FEV1,% | BMI, SD | Oxygen-Therapy | Antibiotic/Anti-Inflammatory Therapy | Insulin Dose, U/kg/day | Transplantation Criteria | |
| CF diagnosis | ||||||
| 16 years | 106/101 | – | No | No/No | 0 | No |
| 17 years | 73/52 | – | No | Yes/Yes | 0 | No |
| 18 years | 61/38 | 19.93 (−0.7) | No | Yes/Yes | 0 | No |
| CFRD diagnosis | ||||||
| 19 years | 57/32 | 18.48 (−1.2) | Yes | Yes/Yes | 0.54 | Yes |
| +4 months | 79/51 | 19.52 (−0.8) | No | No/Yes | 0.51 | No |
| +8 months | 83/53 | 20.49 (−0.5) | No | No/No | 0.45 | No |
| +12 months | 79/50 | 21.17 (−0.2) | No | No/No | 0.41 | No |
| +2 years | 72/45 | 20.81 (−0.5) | No | No/No | 0.39 | No |
| +3 years | 63/30 | 19.49 (−0.8) | No | No/No | 0.43 | No |
| +4 years * | 44/23 | 16.33 (−1.9) | Yes | Yes/Yes | 0.85 | Yes |
| +41/2 years ** | – | 15.95 (−2.1) | Yes | Yes/Yes | 1 | Yes |
| +5 years | 64/35 | 19.68 (−0.9) | No | No/No | 0.77 | No |
| +6 years | 54/31 | 18.75 (−1.1) | No | No/No | 0.78 | No |
| +8 years | 43/47 | 18.72 (−1.1) | No | No/No | 0.76 | No |
CF: cystic fibrosis; CFRD: cystic fibrosis-related diabetes; FVC: forced vital capacity; FEV1: maximal expiratory volume in the first second of forced expiration (FVC and FEV1 expressed in % of the predicted value); BMI (SD): body mass index expressed in kg/m2 and standard deviation; U: units.
*, ** Pulmonary exacerbations.
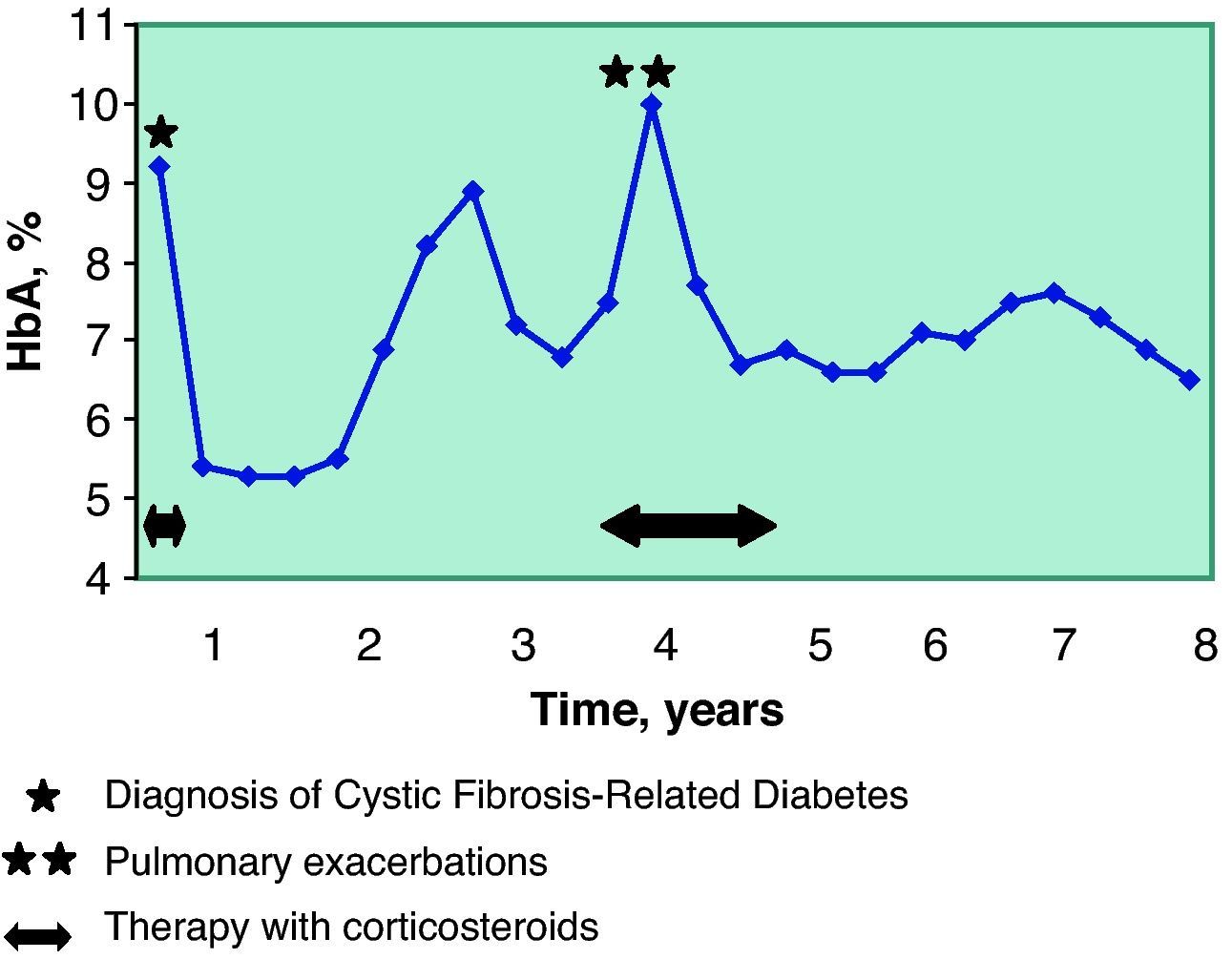
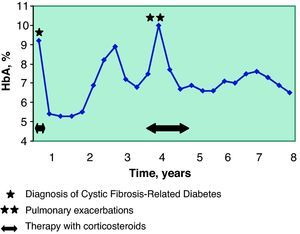
Four years after the diagnosis of diabetes, the patient had another flare-up of ABPA and required oral corticosteroid therapy for 18 months (initial doses of 1.2mg/kg/day and later 0.2mg/kg/day). As a consequence of the immunosuppression caused by the steroids, he presented two pulmonary exacerbations, therefore during this period both the lung function and metabolic control worsened (Table 1, Fig. 1). Once said processes were controlled, all the parameters improved. Since then, the patient has not again needed either antibiotic or corticosteroid treatment.
The control of the diabetes was good from the start of the therapy, with an initial insulin dose of 0.5U/kg/day and adequate levels of HbA1c (Fig. 1). This control experienced alterations coinciding with the lung exacerbations, and it was necessary to increase the dose of insulin to 0.85–1U/kg/day. Afterwards, the patient recovered good glycemic control with a mean insulin dose of 0.7U/kg/day.
Currently, at the age of 27, the patient's CFRD is controlled with the administration of 3 doses of regular insulin before the 3 main meals, together with 2 doses of delayed-action insulin analog detemir (Levemir®) before breakfast and dinner (total dose 0.7U/kg/day). The metabolic control is very good (HbA1c 6.5%), presenting no diabetic complications. Eight years after the diagnosis of CFRD, the patient does not meet lung transplantation criteria, maintains an FVC of 2.08l (43%) and an FEV1 of 1.0l (47%), does not require oxygen therapy and has a good quality of life.
DiscussionSeveral studies have emphasized the beneficial effect of insulin on lung function in patients with CFRD, ascribed not only to its normoglycemia effect but also to the anabolic effect itself of insulin.16 The deficit of insulin increases the risk for lung infection, worsens nutritional state and leads to a reduction in intercostal and diaphragmatic muscle mass, all of which are factors that contribute to the declining lung function in CF patients.17 Therefore, very recently the American Diabetes Association, the American Cystic Fibrosis Foundation and the American Pediatrics Society have published a new Consensus-Guide about the screening and treatment of the hydrocarbonated alterations in CF, with the objective of promoting actions as early as possible in order to protect lung function and the nutritional state of these patients.13 The Guide indicates that, in patients with CF, screening should be done annually starting at the age of 10 by means of an oral overload of glucose. The most innovative agreement is the early use of insulin, even in CFRD without fasting hyperglycemia. It is also pointed out that the use of insulin can even be very positive in phases prior to hydrocarbonated alteration, before the diagnosis of diabetes. Another position of this Consensus is not to use oral anti-diabetic medication in these patients with CF. Dietetic changes and physical exercise pay a secondary role in CFRD, where diet should not be restricted and exercise should be adapted to the possibilities of each patient.
In our patient, the good control of CFRD seems to have played a positive role in the improvement of his lung function. A fact that should be kept in mind in this case is that said benefit meant that he no longer met the criteria for lung transplantation. Over the course of the following eight years and to date, there have been no indications for lung transplantation in this patient, except for a limited period of 6 months in the fourth year after CFRD diagnosis, coinciding with the ABPA and pulmonary exacerbations.
CFRD affects 12%–35% of the CF population, and its prevalence increases with age.6,10 Lung transplantation is the only therapy for definitively controlling the final stage of the lung disease in CF, although it has high mortality18 and there are strict criteria for the selection of the candidates.14,15 Our patient presented factors that are more often associated with the development of CFRD, such as age, the use of systemic corticosteroid therapy and a genotype at risk (mutations R553X and ΔF508).3,7 On the other hand, he had two predictive factors for less severe lung disease: late CF diagnosis and pancreatic exocrine sufficiency (which usually exist in patients with less severe CF mutations and without diabetes). Nevertheless, these factors did not protect him from a rapid deterioration in his lung function. Worsened lung function has been demonstrated months and even years before diagnosis of CFRD,7,8,11 a fact which has been confirmed in all age groups.19
Although the ABPA in this patient before the diagnosis of CFRD could be considered a risk factor for the abrupt respiratory deterioration, the good response of the patient to steroid treatment, made evident by the significant decline in the total serum IgE values, makes it unlikely that ABPA is the main cause of the pulmonary deterioration that the patient experienced. The absence of important side effects of corticosteroid therapy, such as myopathy, also leads us to believe that the corticosteroid therapy also was not an important risk factor that would explain the rapid lung deterioration of the patient. Furthermore, oral steroids, due to their powerful anti-inflammatory action, are occasionally used in the advanced stages of this disease in an attempt at stopping the respiratory deterioration, despite their possible side effects, such as increased pulmonary exacerbations and hyperglycemia. On the other hand, in this patient the pulmonary exacerbations responded positively to antibiotics, but the functional deterioration did not. The coincidence in time of the diagnosis of CFRD and the initiation of insulin therapy with the noticeable improvement in lung function make diabetes the main candidate for the causal factor of the severe respiratory function deterioration, and the insulin is the main candidate for the causal factor of the marked and maintained clinical recuperation of the patient. Along these lines, previous studies have demonstrated significant improvements in pulmonary function during treatment with insulin in patients with CFRD.5,10,11,17 The need to maintain insulin treatment in order to achieve good metabolic control once the pulmonary exacerbations are overcome confirms the diagnosis of diabetes, excluding the corticosteroid treatment as the only cause of the initial hyperglycemia, even more so when the dose of methylprednisolone was low at the moment of the diagnosis of CFRD. Although there are no prospective studies on the duration of the beneficial effect of insulin in CF, the results of the clinical parameters in the evolution of our patient support the idea that, at least in some CF patients, insulin therapy leads to a maintained improvement of the lung disease.20
The genotype–phenotype correlation in CF has been studied for the majority of the clinical characteristics of the disease, and a significant direct correlation has not been found.21,22 Our patient possesses the two most frequent mutations of the CFTR gene associated with diabetes in CF (ΔF508 and R553X). The high prevalence of this genotype in CF goes against a causal relationship with the exceptional evolution that we present here. Furthermore, in an extensive international cohort of 399 patients with CF, the genotypic combination ΔF508/R553X did not correlate with a poor evolution of the lung disease.21 Thus, it is possible that other genes other than CFTR can influence in the pulmonary evolution of patients with CF.23,24
In conclusion, the clinical evolution of our patient supports what has already been reported about the role that insulin and the good metabolic control of CFRD can play in the lung function and nutritional state of these patients. This upholds the need to regularly screen for alterations in the hydrocarbonated metabolism of patients with CF that experience a decline in lung function of unclarified causes, as early treatment of CFRD with insulin can have a positive impact.
Please cite this article as: Martín-Frías M, et al. Efecto beneficioso y prolongado del buen control metabólico de la diabetes relacionada con fibrosis quística sobre la función pulmonar y el estado nutricional. Arch Bronconeumol. 2011;47:532–5.