The diagnosis of latent tuberculosis (LTB) in patients with rheumatoid arthritis (RA) has become important with the introduction of anti-tumor necrosis factor (anti-TNF-α) agents and the appearance of active tuberculosis cases in these patients. The tuberculin skin test (TST) has limited value in patients with RA. Tests based on the release of interferon-gamma (IFN-γ) are being studied, but their role has not been well established for this group of patients.
ObjectivesTo compare the diagnosis of LTB in patients with RA by using cellular immune response to the TST and T.SPOT-TB. Additionally, findings of tomography studies compatible with LTB were used.
MethodsClinical evaluation, TST, T.SPOT-TB and high-resolution computed tomography (HRCT) in a group of patients with RA at the University Hospital of the Federal University of Goiás.
ResultsResponse to the TST was lower in patients with RA (13.5%) compared to the predicted values of the general population. T.SPOT-TB identified a higher number of patients with LTB than the TST (36.8%). HRCT showed changes compatible with LTB in 52.9% of the patients, including 8 of the 11 patients with negative TST and T.SPOT-TB.
ConclusionsThe TST by itself is insufficient to diagnose LTB. A higher number of positive results were obtained with T.SPOT-TB when compared to the TST. Nevertheless, it was negative in a large percentage of patients with tomography findings consistent with LTB. HRCT is readily available in most large health-care centers and it could be incorporated into the diagnostic strategy for LTB in patients with RA.
El diagnóstico de la tuberculosis como infección latente (TBL) en pacientes con artritis reumatoide (AR) ha cobrado importancia con la introducción de agentes anti-factor de necrosis tumoral alfa (anti-TNF-α) y la aparición de casos de tuberculosis activa en estos pacientes.La prueba cutánea de la tuberculina (PT) tiene un valor limitado en pacientes con AR. Se están estudiando las pruebas basadas en la liberación de interferón-gamma (IFN-γ), pero su papel no ha sido bien establecido para este grupo de pacientes.
ObjetivosComparar el diagnóstico de TBL en pacientes con AR con respuesta inmune celular a la PT y T.SPOT-TB. Además, determinar los hallazgos de un estudio de tomografía compatibles con TBL.
MétodosEvaluación clínica, PT, T.SPOT-TB y tomografía computarizada de alta resolución (TCAR) en un grupo de pacientes con AR del Hospital de la Universidad Federal de Goiás.
ResultadosLa respuesta a la PT fue inferior en los pacientes con AR (13,5%), en relación a lo esperado en la población general. El T.SPOT-TB identificó un número mayor de pacientes con TBL al compararlo con la PT (36,8%). La TCAR mostró cambios compatibles con TBL en el 52,9% de los pacientes, incluyendo 8 de los 11 pacientes con PT negativo y T.SPOT-TB.
ConclusionesLa PT por sí misma no es suficiente para diagnosticar la TBL. Un mayor número de resultados positivos se obtuvieron con el T.SPOT-TB, si se lo compara con la PT, aunque fue negativo en un gran porcentaje de pacientes con hallazgos consistentes entre la tomografía y la TBL. La TCAR está disponible en la mayoría de los grandes centros y podría ser incorporada en la estrategia para el diagnóstico de TBL en pacientes con AR.
Tuberculosis (TB) is still a world-wide health problem, and it predominantly affects the segment of the population that is economically active. Brazil is one of the 22 countries where 90% of the world's TB cases are found.1,2 Although the diagnosis and treatment of active tuberculosis is high on the list of priorities of programs aimed at controlling this disease in countries that have a high incidence of TB, latent tuberculosis (LTB) is growing in importance in patients at greater risk for presenting an evolution towards the active form of the disorder. LTB is found in individuals who are believed to host live Mycobacterium tuberculosis germs but do not show any clinical symptom or sign of any type that indicates active disease.3
Since anti-tumor necrosis factor-α (anti-TNF-α) has started to be used in the treatment of rheumatoid arthritis (RA) and other inflammatory diseases, the test for detecting LTB in patients who are candidates for these treatments has taken on a crucial role. Despite the fact that many studies have been done, it still is not clear which is the best approach to use. Several countries have published guidelines in this regard through medical societies, and the majority of them include the histories of contact with active tuberculosis, the result of the tuberculin skin test (TST) and chest radiography images.4,5 After the introduction of the new diagnostic tests for TB, interferon-gamma release assays (IGRA), recent studies have tried to establish their usefulness in patients with RA.
Studies which have compared the result of the TST in patients with RA and in controls have shown significant differences in the results, with an attenuated response in the presence of RA.6,7 Thus, the tuberculin skin test does not seem to be the ideal diagnostic test for LTB in patients with RA, and the evaluation of new strategies has taken on even more importance. The main objective of this study was to evaluate the role of high-resolution computed tomography (HRCT) of the chest for identifying images compatible with LTB. Furthermore, the study evaluated and compared the performance of the tests used to evaluate the cellular immune response (TST and T.SPOT-TB) in the diagnosis of LTB in a group of patients with RA.
Patients and MethodsParticipantsA descriptive study was carried out with the inclusion of 37 consecutive patients from the ambulatory rheumatology clinic at the UFG teaching hospital. In order to be included in the study, the patients needed to be over the age of 18 and have a confirmed diagnosis of RA. The exclusion criteria were suspected pregnancy or confirmed pregnancy, positive HIV serology, or not having attended the appointment for the TST test or for the TST reading.
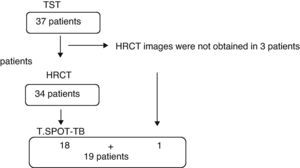
ProceduresThe medical histories for the 37 patients were made available, including the previous diagnosis or contact with individuals with tuberculosis and the administration of BCG. All patients underwent tuberculin skin tests, and in 19 blood samples were extracted in order to obtain peripheral blood mononuclear cells (PBMC) and for the T.SPOT-TB assay (depending on its availability). Thoracic high resolution computed tomography (HRCT) images were obtained in 34 patients (three patients did not keep their HRCT appointment). All the patients included in the study signed an informed consent form. The study was approved by the internal review council of the UFG teaching hospital (protocol number 195/07).
Tuberculin Skin TestThe patients were injected with 100μl (2IU) of PPD Rt 23, using the Mantoux method. The induration was determined 72h after the injection. The same professional was in charge of the administration and the reading of all the tests. An induration ≥5mm was considered to be evidence of reactivity. The person who read the TST did not know the results of the T.SPOT-TB or HRCT.
T.SPOT®-TB (Oxford Immunotec)The test was done following the guidelines provided by the manufacturer. After obtaining the PBMCs, these were prepared and incubated with tuberculosis-specific antigens in a plate prepared with anti-IFN-γ antibodies. If antigens are identified and IFN-γ is produced, it is captured by the antibodies and stains are formed on the lower part of the plate.
High-Resolution Computed TomographyA six-channel multi-cut scanner was used (Somatom Emotion 6, Siemens) in order to obtain the HRCT images of 34 patients. The explorations were assessed by a qualified radiologist with experience in interpreting chest CT images. The evaluator was blind to the results of the TST and of the other tests. The subjects were classified as either having or not HRCT images compatible with LTB, depending on whether they presented one of the following alterations8:
- •
calcified nodules in the pulmonary parenchyma (Ghon nodules);
- •
calcified hilar lymph nodes;
- •
apical pleural thickening;
- •
fibrous lesions in the upper lobes, with or without reduction of the lobe volume.
Fisher's exact test was used for the comparison of the TST and T.SPOT-TB results in the patients who had been vaccinated with BCG versus those who not had received the vaccination. The kappa value was calculated to evaluate the agreement between the results of the TST and the T.SPOT-TB. P values lower than .05 were considered statistically significant.
ResultsIncluded for study were a total of 37 patients (33 women and 4 men) who met the diagnostic criteria of RA from the American College of Rheumatology.9 Mean age was 55.4 (39–81). The time after diagnosis oscillated between 1 and 35 years, with a mean of 11.3 years. Only 3 patients were not being treated with corticosteroids. No patients had received previous tuberculosis treatment. The most important clinical data are described in Table 1.
Clinical Characteristics of the 37 Patients Included.
| Women/men | 33/4 (89%/11%) |
| Mean age (Min–Max) | 55.4 (39–81 years) |
| Mean time since diagnosis (Min–Max) | 11.3 (1–35 years) |
| Current smokers and ex-smokers | 19 (51.3%) |
| Use of corticosteroids at doses >7.5mg of prednisone or equivalent | 16 (43.2%) |
| Use of a drug that could modify the course of the disease (methotrexate/leflunomide or azathioprine) | 29 (78.3%) |
| Use of corticosteroids at doses >7.5mg of prednisone or equivalent or drugs that modify the course of the disease | 34 (91.9%) |
| Administration of BCG | 18 (48.6%) |
Min: minimum, Max: maximum, BCG: Bacillus Calmette-Guérin.
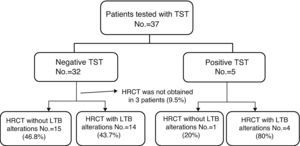
Fig. 1 indicates the procedures done. Out of the 37 patients in the study, 13.5% presented a positive reaction, while 86.5% presented no reaction to TST. Eighteen (48.6%) had been vaccinated with BCG and 16 (51.4%) had not been vaccinated. There were no significant differences for the positive response to TST when we compared the group of patients that had been administered BCG with those who had not received it (P=.39). It was not possible to determine if three of the patients had been vaccinated. These were patients who affirmed having received BCG, but who did not present the characteristic scar that it produces.
Out of the 19 patients tested with T.SPOT-TB assay, 36.8% (7/19) presented a positive reaction and 63.2% presented no reaction. The results of the TST and T.SPOT-TB coincided in 14 patients (73.6%). In 4 patients, the tuberculin skin test was negative and the T.SPOT-TB reactive, and in 1 patient the TST was positive and the T.SPOT-TB negative. There was a reasonable agreement between the two tests (kappa=0.379).
In 34 patients, HRCT images were taken. In 18 patients, there were alterations suggesting LTB and in 16 there were either normal images or alterations unrelated with LTB, like emphysema or interstitial pneumopathy associated with RA. Out of the 5 patients with positive tuberculin test, in 4 the HRCT images were compatible with LTB, and in one they were not. Out of the 32 patients with a negative TST test result in whom HRCT images were obtained, 14 patients presented alterations suggestive of LTB (Fig. 2).
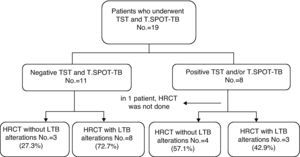
Upon evaluating the group in which T.SPOT-TB was also available (n=19), out of the 11 patients with negative responses to both tests, eight presented alterations suggestive of LTB (Fig. 3 and Table 2).
Alterations on HRCT Images of Each of the 8 Patients with Negative PPD Test and T.SPOT-TB Results and HRCT Images Compatible with LTB.
| Patient | Alterations in HRCT images |
| 1 | Calcified nodule in LUL |
| 2 | Fibrous lesions in LUL+calcified mediastinal lymph nodes |
| 3 | Fibrous pleural alterations and alterations in bilateral apical scarring, with distorted architecture+bronchiectasis+calcified nodule in RUL |
| 4 | Calcified nodule in RLL+calcified lymph node in right hilum |
| 5 | Fibrous pleural alterations and apical scarring+calcified nodule in RUL+calcified mediastinal lymph node |
| 6 | Bilateral apical thickening+parenchymal bands in the lingula+calcified nodule in the ML+calcified mediastinal lymph node |
| 7 | Calcified nodule in RLL |
| 8 | Calcified nodule in RLL |
HRCT: high-resolution computed tomography; LUL: left upper lobe; RUL: right upper lobe; LM: middle lobe; RLL: right lower lobe.
In our study, we have observed a positive tuberculin test in 13.5% (n total=37) of the patients evaluated. Our results are similar to those obtained in other studies. In a study done in Brazil by Marques et al., there were observed positive responses to TST in 14% of the patients with RA, and in 33.3% of the individuals of the control group (patients without RA).10 In Peru, Ponce de Leon et al.7 observed a positive response to the tuberculin test in 29% of the population with RA, compared with 71% in the control group. In Argentina, Tamborenea et al.11 observed positive responses to TST in 12.4% of a cohort of patients with RA.
Several researchers coincide in arguing that the TST is less able to diagnose LTB in patients with RA, compared with healthy individuals.7,10,11 The mechanism that causes attenuated TST response in patients with RA is not completely clear, but it is related with an anomalous cellular immune function observed in these patients.12–14 Some studies have demonstrated that the tuberculin skin test is more sensitive in patients with certain types of immunosuppression, such as that found in RA, when using higher doses of TST.15–17
In this study, the positive responses to the tuberculin skin test were not affected by the history of BCG administration. The influence of the BCG vaccine in the TST result has been known for a long time; however, recent studies have considered this effect to be minimal.18
In our study, the T.SPOT-TB assay was reactive in 36.8% of the patients evaluated, compared with 21% with positive TST responses in this group. In several previous studies, higher positivity of IGRA has been demonstrated when compared with tuberculin skin tests for the diagnosis of LTB in immunosuppressed patients. The results of this study regarding T.SPOT-TB are similar to those of other studies comparing IGRA and TST in patients with RA, and they reveal that the former method produces more positive results.10,19,20 Nevertheless, in a patient with positive TST, the T.SPOT-TB was not reactive. This patient presented alterations on CT that were compatible with LTB, which suggests that T.SPOT-TB produced a false negative result. In one recent study done in patients with intestinal inflammatory diseases in whom tests were done for detecting LTB for the use of anti-TNF-α, the majority of whom were being treated with immunomodulators (corticosteroids, methotrexate or anti-TNF treatment), it has been demonstrated that the performance of the IGRAs is negatively affected by the use of immunomodulators (OR 0.3, 0.1–0.9).21 In our study, the majority of the patients (91.9%) had been prescribed an immunosuppressant and/or a dose of corticosteroids of more than 7.5mg of prednisone or its equivalent.
Several studies have suggested that IGRAs have a probability for giving false positive results less than that of TST in diagnosing LTB. However, it is worthy to note that Mycobacterium leprae, the etiological agent of Hansen's disease (leprosy), which is endemic in Brazil, has a homologous antigen for the ESAT-6 used in IGRAs: L-ESAT-6. In a collaboration study in which researchers from Brazil and Holland participated, it was demonstrated that there was a cross-reaction between these antigens, which could limit their use as a diagnostic tool in tuberculosis and Hansen's disease in regions where these two diseases are endemic.22
There were a significant number of patients with negative TST and T.SPOT-TB results who were therefore classified as patients without LTB, but whose CT images showed alterations compatible with LTB. The role of thoracic radiography in the diagnostic study of LTB in patients with AR in whom the use of anti-TNF-α drugs is indicated has already been clearly established. Several guidelines affirm that chest radiography is essential, and if the presence of any alteration suggestive of LTB is determined, the treatment of the infection is indicated.4,5
The greater sensitivity of HRCT compared with chest radiograph images has been widely documented in the international literature. This method has gained greater acceptance as an excellent diagnostic instrument, especially in selected cases, such as patients with respiratory symptoms and negative sputum smear, possible cases of tuberculosis in which the image of the thoracic radiograph is either normal or inconclusive, patients in whom an examination of the mediastinum is necessary, those with disseminated disease or those who present endobronchial alterations.23 In previous studies, it has already been seen that HRCT is superior to chest radiography for detecting alterations that suggest LTB or active TB during tuberculosis breakouts or among contacts at home. In a study performed during a tuberculosis breakout at a school in South Korea, the researchers ran tuberculin skin tests and ordered chest radiographs for all the students and staff. In those individuals with positive tuberculin skin tests, the QuantiFERON-TB-GOLD was applied and thoracic CT images were taken in those cases where QuantiFERON-TB-GOLD was positive or TST was >20mm. Out of the 52 individuals with thoracic CT images, radiography was normal in 46. Thoracic CT showed that 11 (23.9%) and 7 (15.2%) of these individuals presented alterations that suggested active TB and LTB, respectively.24 In a study done during an endemic breakout of tuberculosis in soldiers stationed in South Korea, 87 individuals were included and 18 of these were diagnosed with TB. Nine of these patients presented normal chest radiography but showed lesions suggestive of active TB on HRCT.25 In our study, the use of HRCT was evaluated as a possible diagnostic tool for LTB in patients with RA. As we still do not have a diagnostic method for LTB that constitutes a reference pattern and as the methods that have been introduced still provide contradictory results in patients with RA, HRCT may be a useful option for reaching a diagnosis. It has been demonstrated that we still do not have available a test with a sensitivity of 100% for the diagnosis of LTB; consequently, it is possible that the combination of various tests constitutes the best option. In this study, 72.7% (8/11) of the patients with negative TST and T.SPOT-TB results presented alterations suggesting the presence of LTB on HRCT. It should be mentioned that alterations in lung structures may be associated with RA, caused either by a defect in immunity or the chronic inflammation produced by the disease itself, or even because of the medication used to treat RA, which can cause lung toxicity. The most frequent manifestations are interstitial pneumopathy, pleural hemorrhage or thickening, lung nodules and respiratory tract disease.26 Only calcified nodules are considered indicative of LTB, as they are very infrequent in rheumatoid arthritis. Moreover, in all cases of pleural thickening, calcified nodules were also observed. In spite of the fact that other infectious granulomatous diseases, such as histoplasmosis, can leave residual lesions similar to those caused by tuberculosis, the majority of the epidemiological research done in Brazil suggest that the prevalence of histoplasmosis is considerably less than that of tuberculosis in most of the country.27 Thus, the alterations observed on HRCT probably reflect previous contact with the tuberculosis bacillus that left pulmonary side effects which the other tests (TST and T.SPOT-TB) were not able to identify. Consequently, in this group of patients with false negative results for tuberculosis on the tuberculin skin test and T.SPOT-TB, treatment for LTB should be contemplated before the use of anti-TNF-α drugs. More than half of the patients with positive TST and/or T.SPOT-TB presented normal HRCT. This observation shows the importance of using diagnostic methods that complement each other. HRCT should be used as an additional exploration, not as a substitution for immunological tests.
Our study has certain limitations. This first is the lack of a control group made up of patients without RA. Such a group would allow us to analyze differences in the response to TST and T.SPOT-TB. The second is the lack of a diagnostic method that could be considered a reference pattern for the diagnosis of LTB, with which it would be possible to more adequately evaluate the sensitivity and specificity of the different diagnostic strategies. Another limitation is that not all the patients of the RA group were examined with the three tests (TST, T.SPOT-TB and HRCT) and it is possible that that has caused a selection bias. Nevertheless, we believe that this did not cause an important bias as the patient selection for the application of the three tests was done randomly, depending on the availability of said tests.
In conclusion, to date, most studies indicate that the tuberculin skin test is insufficient as a diagnostic study of LTB in patients with RA. The new diagnostic methods for LTB have not been completely validated in this group of patients in populations with different rates of incidence of the disease. In addition, they are still not recommended as part of standard clinical practice in most countries. Thoracic HRCT is a widely available technique in large hospital centers and could be used as an additional tool for the diagnosis of LTB.
FundingThis article received funding from the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), n. 475603/2007-9.
Conflicts of interestThe authors declare having no conflicts of interest.
Please cite this article as: Tannus Silva DGS, et al. Tuberculosis latente en la artritis reumatoide. Evaluación de la respuesta celular y tomografía computarizada de alta resolución. Arch Bronconeumol. 2012;48:144–9.