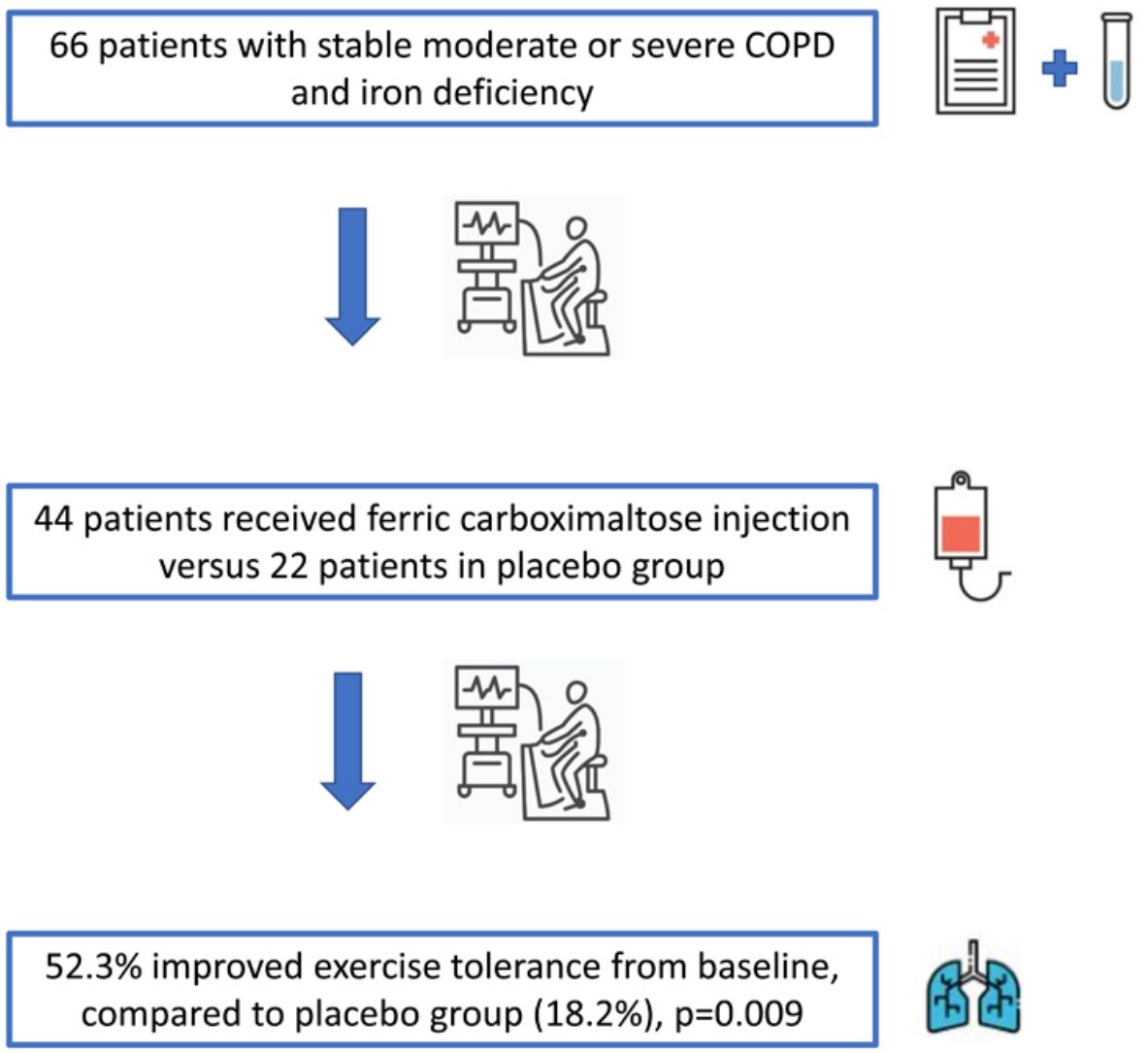
Iron deficiency affects exercise capacity because of the critical role iron plays in the optimal functioning of skeletal muscle metabolism. We hypothesized that intravenous iron may improve exercise tolerance, quality of life (QoL), and daily physical activity (DPA) in patients with chronic obstructive pulmonary disease (COPD).
MethodsThis was a placebo-controlled, single-blind, parallel-group, randomized clinical trial. Iron deficiency was defined as a ferritin level<100ng/mL or a ferritin level between 100 and 299ng/mL with a transferrin saturation<20%, with or without mild anaemia. Patients were randomized at a 2:1 ratio to receive intravenous ferric carboxymaltose or placebo. The primary objective was to investigate whether intravenous iron replacement improved endurance time from baseline by at least 33%. The secondary objectives were to evaluate impact on QoL using the COPD Assessment Test (CAT) and on DPA by accelerometry.
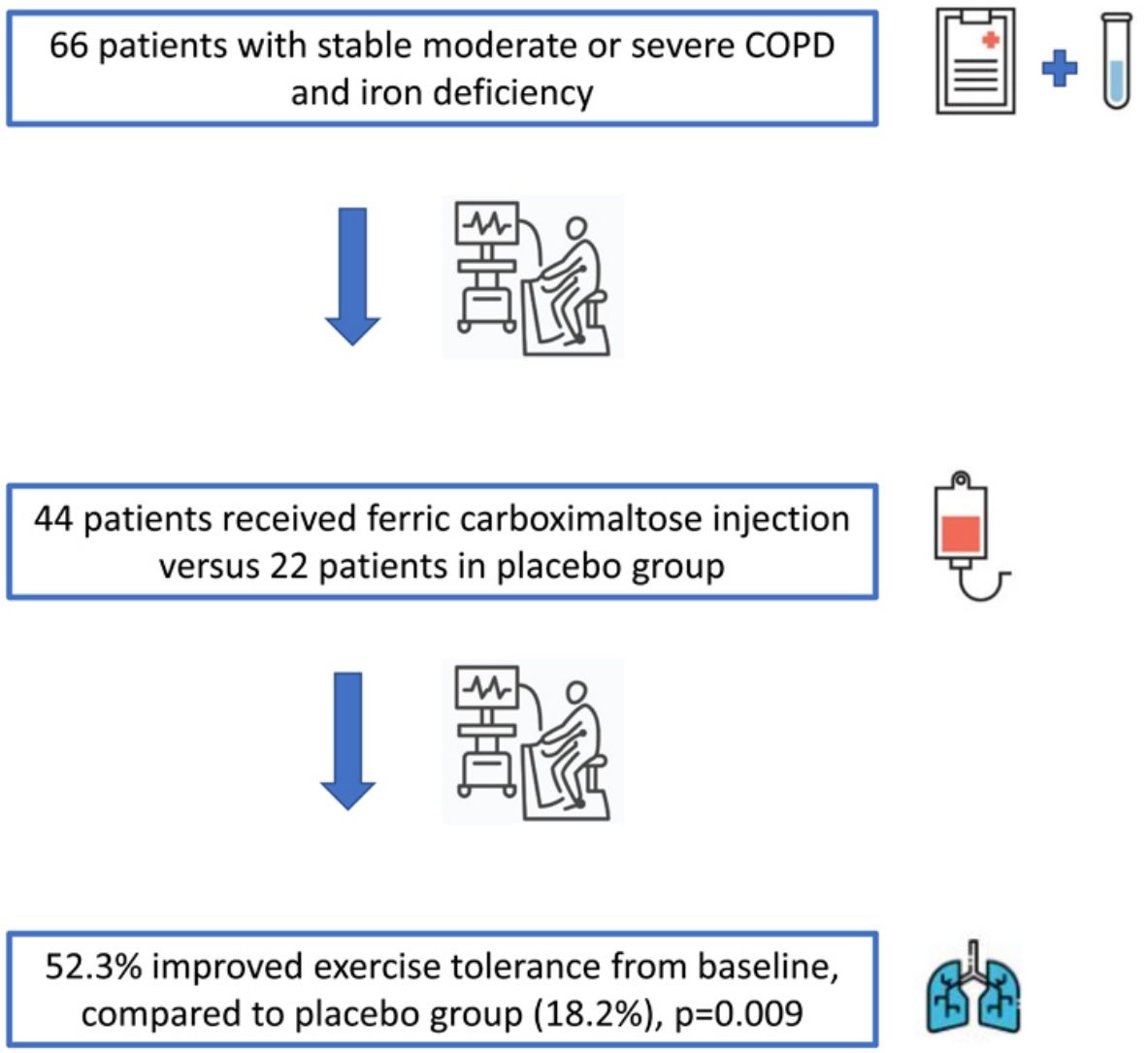
ResultsWe included 66 patients, 44 (66.7%) in the intervention group and 22 (33.3%) in the placebo group. Among patients receiving ferric carboxymaltose, 23 (52.3%) achieved the primary endpoint compared to 4 (18.2%) in the placebo group [p=0.009; relative risk 3.12, (95% CI, 1.19–8.12)]. CAT score decreased −3 (−6.0–1.3) points from baseline in the intervention group (p=0.007), in contrast to placebo group [−1 (−4.0–2.3) points, p=0.236] with no differences in DPA and adverse events in both groups.
ConclusionsIron replacement improved exercise capacity and QoL in stable COPD patients with iron deficiency. The treatment was well tolerated.
Clinical Trial registrationEudraCT 2016-001238-89.
Chronic Obstructive Pulmonary Disease (COPD) is a major cause of morbidity and mortality worldwide.1,2 COPD is associated with various comorbidities such as cardiovascular disease, cachexia, osteoporosis, metabolic syndrome, depression, pulmonary hypertension, lung cancer, and anaemia.1–3 These comorbidities influence mortality and hospitalizations independently, so should be treated appropriately.2,4 Anaemia is increasingly recognized as an important comorbidity,5 being and independent predictor of dyspnoea, exercise limitation, anaerobic threshold, worse health-related quality of life (QoL), and mortality.6–8 Beyond anaemia, non-anaemic iron deficiency (NAID) has also been linked to worse clinical outcomes including frequent exacerbations, and predicts exercise intolerance.6,8
Iron is an essential micronutrient in energy metabolism9 since it plays a key role in oxygen transport (as part of the ferrous ring of haemoglobin (Hb)), storage (as a component of myoglobin), uptake in the oxidative metabolism within the skeletal muscle and in cellular bioenergetics as a whole.10–12 In other words, cellular oxidative metabolism strongly relies on iron availability, which is indispensable for both sufficient oxygen supply, mitochondrial function, and effective substrate catabolism.9 Furthermore, iron is also involved in erythropoiesis.12 Therefore, an adequate iron homeostasis is crucial for cell activity, especially for those characterized by intensive metabolism and high energy demand, such as skeletal myocytes and cardiomyocytes,13 which are key elements in skeletal muscle and heart functions, respectively. In this regard, iron deficiency (ID) may significantly contribute to the loss of skeletal muscle oxidative capacity seen in patients with chronic heart failure (CHF) and COPD.9 Therefore, peripheral and respiratory muscle dysfunction may occur, constituting a potential pathophysiological link between impaired iron status and decreased exercise capacity.14
In recent years, ID with or without anaemia has been studied in pulmonary arterial hypertension (PAH), CHF, and chronic kidney disease, where it was associated with impaired exercise tolerance and worse clinical outcomes.11,13,15 In a previous investigation from our group, COPD patients with NAID showed lower pre-training aerobic capacity and reduced training-induced response in comparison with those with normal iron status, suggesting that ID hampered pulmonary rehabilitation in COPD patients.16 Surprisingly, even though iron supplementation is associated with clinical benefits in patients with PAH or CHF,11,13,15 the impact of this therapy in COPD has not been fully elucidated. In the only randomized clinical trial published to date, intravenous iron supplementation did not show any effect on arterial oxygenation, although it increased exercise capacity improving the six-minute walk test (6MWT).17
The FACE (‘Ferinject Assessment in patients with COPD and iron deficiency to improve Exercise tolerance’) study was designed to test the hypothesis that, compared to placebo, intravenous iron repletion would improve exercise tolerance in patients with ID. Secondary objectives included the assessment of potential changes in QoL and daily physical activity (DPA).
MethodsTrial designFACE was a single-blind, parallel group, placebo controlled clinical trial conducted in a single tertiary care hospital. Patients included were from January 2018 to January 2020 and were subsequently randomized with a 2:1 sequence to receive a single dose of ferric carboxymaltose or placebo. The study methods have been fully detailed in the supplementary material.
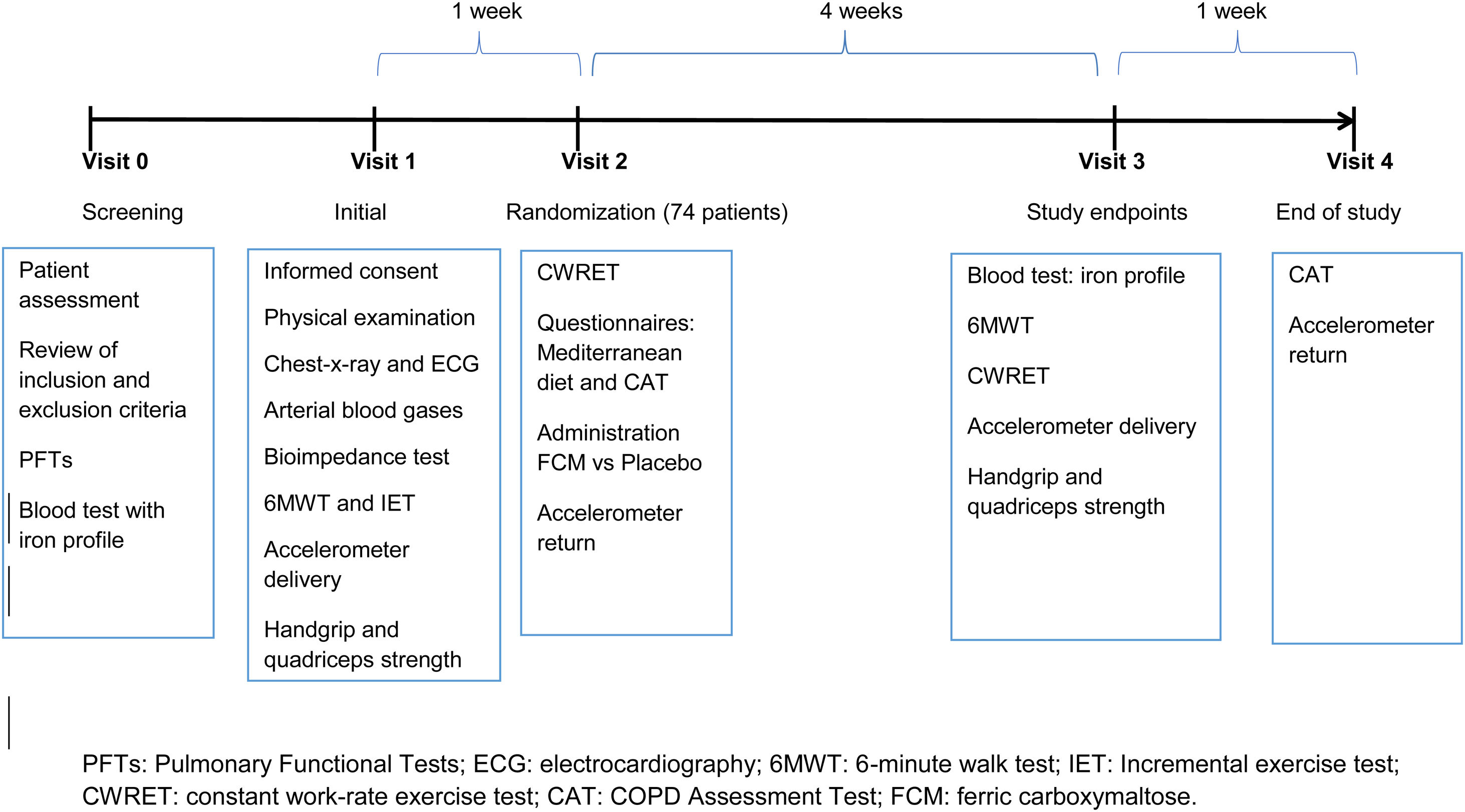
ParticipantsEligibility criteria, recruitment, and follow-upEligible participants were adults aged between 45 and 80 years, diagnosed of COPD according to Global Strategy of Management of COPD patients (GOLD) criteria.18 Patients must have shown clinical stability for at least 8 weeks prior to their inclusion in the study. Patients were recruited from primary, secondary and tertiary care settings. ID and mild anaemia definitions are included in the supplementary material. The study flow chart including the follow-up is summarized in Fig. 1. Patients signed the informed consent that indicated that they had been informed of all pertinent aspects of the trial in visit 1.
Randomization, study therapy and blindingIn visit 2, randomization was performed by a clinical pharmacist according to a computer-generated list following simple randomization procedures to receive either ferric carboxymaltose (Ferinject® 50mg/ml solution for intravenous injection, Vifor, Switzerland) or placebo. Full details of the trial blinding can be found in the supplementary material.
OutcomesThe primary outcome was the achievement of at least 33% improvement from baseline in the endurance time (ET) obtained during a constant work-rate exercise test (CWRET) using cycle-ergometer.19 Secondary endpoints included: a COPD Assessment Test (CAT)20 and DPA, evaluated by accelerometry. Conventional laboratory parameters were also performed. The strength of the upper (handgrip) and lower limb (quadriceps maximal velocity contraction, QMVC) muscles was measured at baseline and following treatment in all the study patients.21 All the outcomes were measured at baseline and 4 weeks after treatment administration, and potential clinical adverse events were recorded and reported, including anticipated and unexpected circumstances according to CONSORT extended guidelines.22
Statistical analysisAccording to a previous study,19 to detect at least a 33% [95% confidence interval (CI), 18–48%] ET with a two-sided 5% significance level, a statistical power of 80% and a dropout rate of 20%, a sample size of 42 patients (28 in the ferric carboxymaltose group and 14 in the placebo group) was deemed necessary.19
Due to the predefined sample size, non-parametric tests were employed to perform the analysis. Exact methods used to compare groups and subgroup analysis were detailed in the supplementary material. A p<0.05 was considered statistically significant in all the analyses, which were performed with the IBM SPSS Statistics 22.0 package (IBM Co., Armonk, NY).
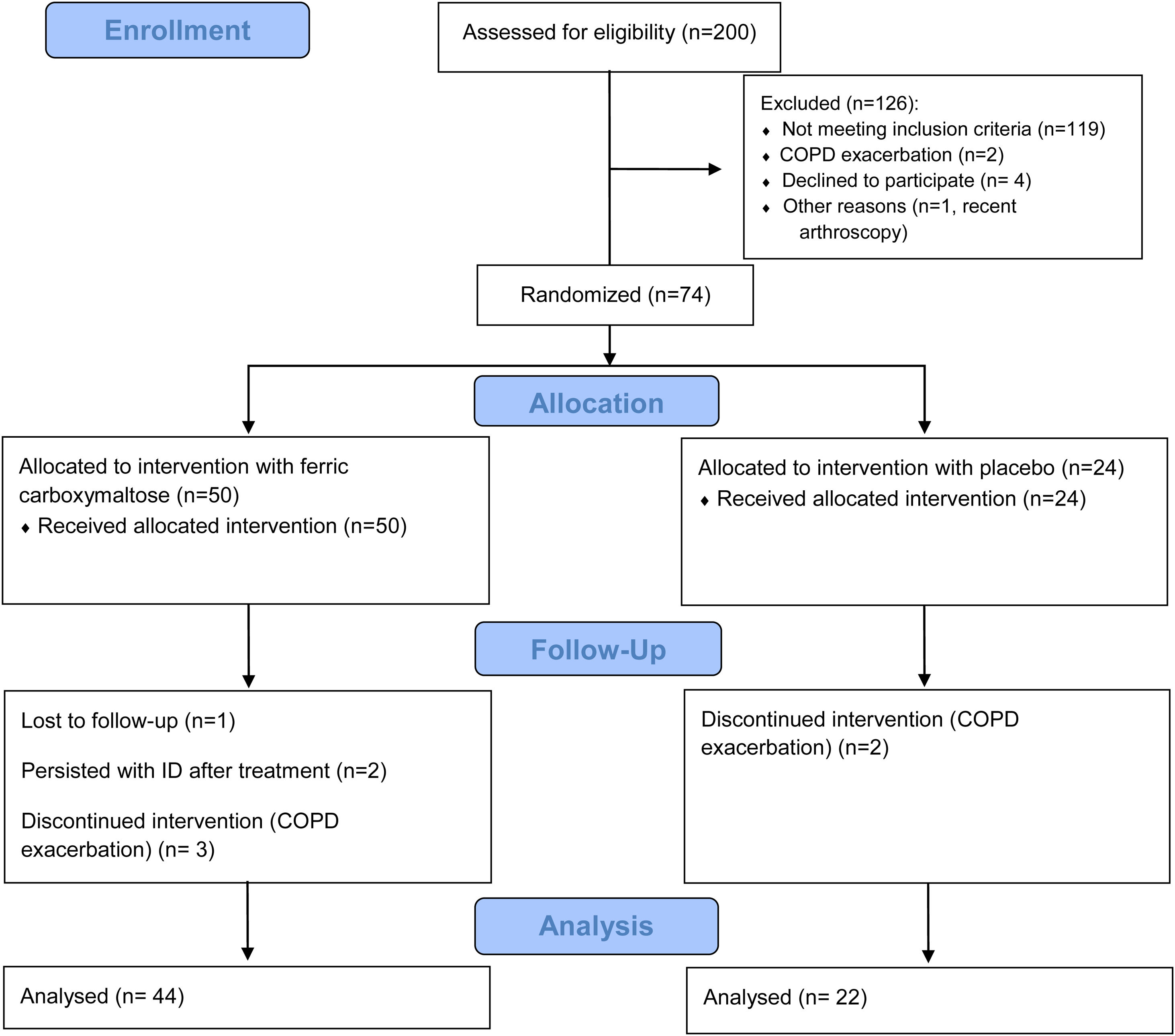
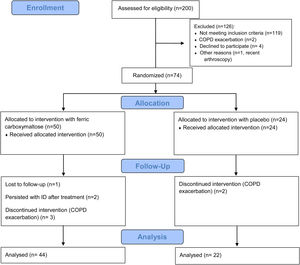
ResultsThe study flow diagram is detailed in Fig. 2. Although a total of 200 patients were initially assessed for eligibility, only 74 (37.0%) were randomized (50 in the ferric carboxymaltose group and 24 in the placebo group). However, five patients discontinued the trial due to a COPD exacerbation and one was lost in the follow-up. Two more patients who did not replace ID were also excluded. Finally, a total of 66 (33%) patients [44 (66.7%) in the ferric carboxymaltose group and 22 (33.3%) in the placebo group] were included in the analysis. Interestingly, from the consecutive 200 patients that were initially evaluated, 40.5% showed ID.
Baseline clinical, demographical, and analytical characteristics are described in Table 1. Both groups presented similar baseline characteristics, including in the handgrip and quadriceps strength tests. Median administered ferric carboxymaltose dose was 500 (500–1000) mg.
Baseline clinical, demographical, and analytical characteristics of included patients.
| Ferric carboxymaltose (N=44) | Placebo (N=22) | |
|---|---|---|
| Age, years | 68.0 (62.0–71.8) | 69.5 (66.0–72.3) |
| Sex, female | 18 (39.1) | 6 (27.3) |
| BMI, kg/m2 | 24.8 (21.0–27.0) | 24.9 (23.3–27.6) |
| FFMI, kg/m2 | 15.1 (14.1–17.7) | 16.5 (15.0–17.8) |
| Smoking status | ||
| Past | 20 (45.5) | 13 (59.1) |
| Current | 24 (54.5) | 9 (40.9) |
| Pack-years | 47.5 (32) | 45.0 (25.0–80.0) |
| Mediterranean diet questionnaire | 7.5 (6.0–9.8) | 9.0 (7.0–10.0) |
| Medical history | ||
| Arterial hypertension | 20 (45.5) | 13 (59.1) |
| Diabetes mellitus type II | 8 (18.2) | 4 (18.2) |
| Dyslipidaemia | 19 (43.2) | 7 (31.8) |
| Atrial fibrillation | 3 (6.8) | 1 (4.5) |
| Ischaemic cardiomyopathy | 4 (9.1) | 2 (9.1) |
| Charlson comorbidity index | 4.0 (3.3–5.8) | 4.0 (3.0–5.0) |
| Laboratory analysis | ||
| Creatinine, mg/dL | 0.86 (0.7–1.04) | 0.9 (0.7–1.0) |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 82.5 (68.4–95.0) | 83.5 (68.3–90.2) |
| Total protein, g/dL | 6.9 (6.7–7.4) | 6.9 (6.6–7.2) |
| Albumin, g/dL | 4.5 (4.3–4.6) | 4.5 (4.2–4.8) |
| C-reactive protein, mg/dL | 0.2 (0.1–0.3) | 0.3 (0.1–0.5) |
| ESR, mm/h | 8.5 (4.5–12.0) | 6.5 (2.8–8.3) |
| Fibrinogen, mg/dL | 386.5 (313.5–430.3) | 382.5 (339.5–480.8) |
| Vitamin D, ng/mL | 11.5 (9.0–19.0) | 14.0 (7.8–28.8) |
| Iron status | ||
| Haemoglobin, g/dL | 14.4 (13.7–15.5) | 15.0 (13.9–15.6) |
| Haematocrit, % | 43.9 (40.9–46.5) | 45.6 (41.2–47.6) |
| MCV, fl | 90.7 (88.3–92.9) | 90.6 (86.8–93.3) |
| MCH, pg | 30.2 (29.1–31.2) | 30.1 (29.0–31.3) |
| MCHC, g/dL | 33.1 (32.5–33.9) | 33.2 (32.5–33.5) |
| Serum iron, μg/dL | 77.0 (56.7–109.4) | 78.4 (54.5–108.6) |
| Ferritin, ng/mL | 56.5 (31.5–87.8) | 60.5 (36.8–93.0) |
| Transferrin saturation, % | 20.7 (15.2–28.1) | 20.2 (15.4–28.2) |
| Transferrin, g/dL | 278.0 (245.5–308.8) | 264.5 (245.0–287.3) |
| Soluble transferrin receptor, mg/L | 2.9 (2.1–3.2) | 3.0 (2.1–3.4) |
| Pulmonary function | ||
| FEV1, L | 1.3 (1.0–1.6) | 1.2 (0.9–1.5) |
| FEV1, % predicted. | 55.0 (41.0–60.5) | 47 (38.5–54.3) |
| FVC, L | 2.7 (2.1–3.2) | 2.9 (2.5–3.7) |
| FVC, % | 80.5 (71.0–89.8) | 83.5 (76.0–93.3) |
| FEV1/FVC, % | 49.4 (41.5–57.4) | 43.2 (34.5–50.3) |
| TLC, % | 111.5 (100.0–119.5) | 108.0 (100.3–120.0) |
| DLCO, % | 53.0 (42.3–62.5) | 52.0 (42.8–60.3) |
| Post-bronchodilator | ||
| FEV1, % predicted. | 59.0 (46.3–67.0) | 49.5 (41.8–62.0) |
| FEV1/FVC, % | 51.5 (41.6–59.4) | 47.7 (35.7–53.1) |
| FVC, % | 82.0 (77.0–94.0) | 88.5 (79.0–100.0) |
| Arterial blood gases | ||
| PaO2, mmHg | 75.6 (69.5–82.7) | 81.0 (74.2–82.9) |
| PaCO2, mmHg | 38.5 (36.5–40.8) | 37.3 (35.1–41.1) |
| SaO2, % | 95.0 (93.0–96.0) | 95.0 (94.0–96.5) |
| Cardiopulmonary exercise testing | ||
| Peak Load, W | 68.5 (53.0–80.0) | 73.0 (46.8–87.3) |
| Peak Load, % | 66.0 (48.0–91.0) | 72.0 (54.0–83.5) |
| Peak V′O2/kg, ml/min/kg | 15.6 (13.0–19.5) | 14.9 (12.6–17.8) |
| Peak V′O2, % predicted | 70.5 (50.5–93.5) | 74.0 (60.5–81.5) |
| RQ max | 1.1 (1.0–1.2) | 1.2 (1.1–1.3) |
| V′E max, l/min | 40.3 (30.1–46.9) | 39.5 (31.6–52.0) |
| V′E max, % | 68.0 (54.0–84.0) | 69.0 (56.8–82.3) |
| HR max, l/min | 130.5 (115.3–140.8) | 120.1 (111.8–131.3) |
| HR max, % | 82.0 (75.5–89.8) | 81.5 (74.0–84.5) |
| V′O2/HR max | 8.0 (6.2–9.2) | 8.7 (6.5–10.2) |
| V′O2/HR max, % | 82.0 (65.5–89.5) | 86.0 (73.0–102.0) |
| Oxygen desaturation, % | 0 (0) | 0 (0) |
| V′O2 at AT, ml/min | 677.5 (548.0–786.3) | 551.0 (415.0–694.0) |
| V′O2 at AT, % | 52.0 (45.0–61.3) | 44.0 (38.0–49.0) |
| V′E/V′CO2 at AT | 36.0 (32.0–40.0) | 37.0 (33.5–41.5) |
| Hyperinsuflation, ml | 420.0 (277.5–562.5) | 385.0 (0.0–500.0) |
| Final systemic blood pressure, mmHg | 190.0 (180.3–203.8) | 197.0 (174.5–205.3) |
| Final diastolic blood pressure, mmHg | 90.0 (80.0–102.8) | 84.0 (77.8–106.3) |
| Final dyspnoea Borg score | 5.0 (3.0–7.0) | 6.0 (5.0–7.0) |
| Final leg discomfort (Borg score) | 6.0 (4.0–8.0) | 7.0 (4.0–7.0) |
| Concomitant treatment | ||
| LAMA | 8 (18.2) | 1 (4.5) |
| LABA | 1 (2.3) | 0 (0.0) |
| LAMA+LABA | 19 (43.2) | 7 (31.8) |
| LAMA+ICS | 0 (0.0) | 0 (0.0) |
| LABA+ICS | 2 (4.5) | 1 (4.5) |
| LAMA+LABA+ICS | 14 (31.8) | 13 (59.1) |
| Beta-blocker | 6 (13.6) | 3 (13.6) |
Data are presented as median (Q1–Q3) or as absolute number (percentage).
BMI, body mass index; FFMI, fat free mass index; Glomerular filtration rate was calculated by Chronic Kidney Disease Epidemiology Collaboration; ESR, erythrocyte sedimentation rate; MCV, mean corpuscular (erythrocyte) volume; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; TLC, total lung capacity; DLCO, carbon monoxide diffusion capacity corrected for haemoglobin concentration; PaO2, oxygen partial pressure; PaCO2, carbon dioxide partial pressure; peak V′O2, peak oxygen uptake; RQ max, maximum respiratory quotient; V′E max, maximum minute ventilation; HR max, maximum heart rate; V′O2/HR, oxygen pulse; AT, anaerobic threshold; V′E/V′CO2, ventilatory equivalent for carbon dioxide; LAMA, long-acting antimuscarinic antagonists; LABA, long-acting beta2-agonists; ICS, inhaled corticosteroid.
Mediterranean diet: according to the questionnaire performed by Spanish Society of Obesity, available in https://www.seedo.es/index.php/pacientes/dieta-mediterranea. Date last updated: December 15, 2020. Date last accessed: December 15, 2020.
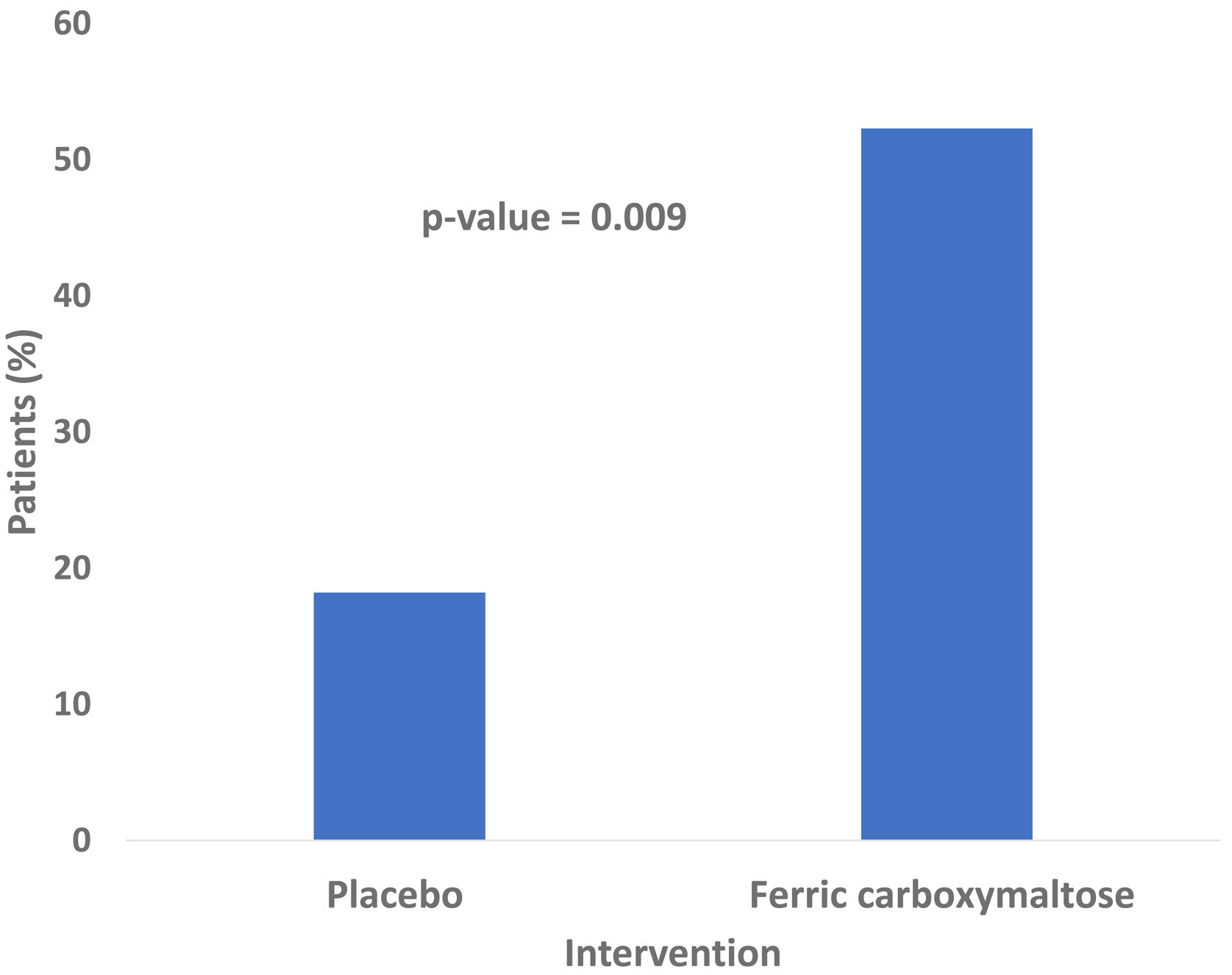
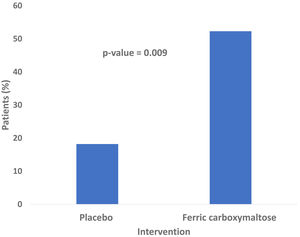
As far as 52.3% of patients improved their ET by 33% in the treatment group compared to only a 18.2% in the placebo group (p=0.009; Relative Risk (RR) 3.12, [95% CI, 1.19–8.12], as shown in Fig. 3). The absolute difference was 34.1% (95% CI, 10.20–53.50). Therefore, the treatment of three patients would achieve the primary endpoint in at least 1 patient. Data on the effects of treatment on ET and other clinical outcomes were shown in Table 2. Regarding the change from baseline in ET, statistically significant differences were found between placebo and ferric carboxymaltose group [119.5 (13.8–273.3)s vs. −8.0 (−50.8–69.0)s, p=0.010)]. The inspiratory capacity in the constant work-rate exercise test was assessed in a total of 46 patients (31 in the ferric carboxymaltose group and 15 in the placebo group). No differences were found after the treatment both in patients treated with intravenous iron (p=0.542) or placebo (p=0.875). Handgrip and quadriceps strength tests were also similar after iron treatment between the two study groups: handgrip strength test (iron group p=1.000, placebo group p=0.506) and quadriceps strength test (iron group p=0.793, placebo group p=0.952).
Comparison of main clinical outcomes in the constant work-rate exercise test (CWRET) among ferric carboxymaltose and placebo groups.
| Ferric Carboxymaltose (N=44) | Placebo (N=22) | p value | |
|---|---|---|---|
| Endurance time, seconds | |||
| Before | 279.0 (245.5–344.5) | 268.5 (240.8–364.5) | 0.927 |
| After | 444.0 (304.0–557.5) | 252.5 (230.8–385.3) | 0.020 |
| Heart rate, bpm | |||
| Before | 121.5 (108.5–140.5) | 117.5 (109.0–128.0) | 0.366 |
| Isotime | 125.0 (105.0–130.0) | 110.5 (105.5–120.0) | 0.231 |
| After | 128.0 (109.5–138.8) | 114.0 (103.0–127.5) | 0.017 |
| Minute ventilation, L/min | |||
| Before | 38.9 (30.2–46.3) | 40.7 (32.5–49.5) | 0.546 |
| After | 39.3 (31.8–52.3) | 40.1 (32.3–49.0) | 0.483 |
| Final dyspnoea (Borg score) | |||
| Before | 5.0 (3.0–7.0) | 6.0 (4.5–7.5) | 0.238 |
| After | 4.0 (3.0–7.0) | 5.5 (4.0–7.0) | 0.187 |
| Final leg discomfort (Borg score) | |||
| Before | 7.0 (5.0–8.0) | 6.0 (4.0–7.0) | 0.308 |
| After | 5.0 (4.0–8.0) | 6.5 (4.8–8.3) | 0.118 |
Data are presented as median (Q1–Q3).
Bpm: beats per minute.
From the 44 patients treated with intravenous iron, 4 (9.1%) presented mild anaemia. These patients ameliorated their ET by 238.0 (78.8–264.5) s, p=0.043, all of them achieving the primary endpoint. No differences were found in the change from baseline in ET compared to patients with NAID [238.0 (78.8–264.5) vs. 103.5 (9.3–278.0), p=0.541] or Hb [1.2 (−0.1–1.8) vs. 0.5 (−0.2–1.2) g/dL, p=0.383].
Iron statusAnalytical parameters before and after treatment or placebo administration were presented in Table 3. The administration of intravenous iron resulted in a significant improvement in all the analytical parameters concerned by this ion metabolism (p<0.001 for all comparisons).
Levels of analytical parameters according to study treatment.
| Ferric carboxymaltose (N=44) | Placebo (N=22) | p value | |
|---|---|---|---|
| Haemoglobin, g/dL | |||
| Before | 14.4 (13.7–15.5) | 15.0 (13.9–15.6) | 0.256 |
| After | 14.9 (14.0–16.0) | 15.1 (14.4–15.8) | 0.910 |
| Change from baseline | 0.6 (−0.2 to 1.2) | 0.3 (−0.3 to 0.6) | 0.036 |
| Serum iron, μg/dL | |||
| Before | 77.0 (56.7–109.4) | 78.4 (54.5–108.6) | 0.911 |
| After | 104.1 (87.0–123.0) | 87.2 (69.5–111.3) | 0.031 |
| Change from baseline | 30.3 (−2.6 to 44.0) | 14.0 (−14.0 to 35.4) | 0.065 |
| Ferritin, ng/mL | |||
| Before | 56.5 (31.5–87.8) | 60.5 (36.8–93.0) | 0.564 |
| After | 306.0 (234.0–432.0) | 51.5 (36.5–93.3) | 0.000 |
| Change from baseline | 216.0 (150.0–368.0) | −4.0 (−13.5 to 11.3) | 0.000 |
| Transferrin saturation, % | |||
| Before | 20.7 (15.2–28.1) | 20.2 (15.4–28.2) | 0.762 |
| After | 32.7 (27.5–40.9) | 22.7 (17.9–29.5) | 0.000 |
| Change from baseline | 12.7 (5.2–19.5) | 3.2 (−6.0 to 7.8) | 0.000 |
| Transferrin, g/dL | |||
| Before | 278.0 (245.5–308.8) | 264.5 (245.0–287.3) | 0.274 |
| After | 232.0 (200.0–248.0) | 281.0 (254.0–303.8) | 0.000 |
| Change from baseline | −42.0 (−70.0 to −22.0) | 10.0 (−5.8–27.3) | 0.000 |
| Soluble transferrin receptor, mg/L | |||
| Before | 2.9 (2.1–3.4) | 3.0 (2.1–3.4) | 0.728 |
| After | 2.4 (2.1–3.1) | 2.7 (2.3–3.5) | 0.132 |
| Change from baseline | −0.3 (−1.0 to −0.03) | 0.07 (−0.08–0.6) | 0.002 |
Data are presented as median (Q1–Q3).
The analysis of these secondary outcomes is summarized in Table 4. There were no statistically significant differences with respect to placebo in the change of the scores of the CAT questionnaire. In patients with mild anaemia treated with intravenous iron, CAT questionnaire improved by −7.5 (−16.5–2.3), p=0.138.
Comparison of secondary outcomes (CAT questionnaire and daily physical activity) between patients treated with ferric carboxymaltose and placebo.
| Ferric carboxymaltose (N=44) | Placebo (N=22) | p value | |
|---|---|---|---|
| CAT questionnaire | |||
| Before | 14.5 (9.8–20.3) | 14.0 (7.8–21.0) | 0.738 |
| After | 9.5 (5.8–18.3) | 12.5 (9.0–17.3) | 0.415 |
| Change from baseline | −3.0 (−6.0 to 1.3) | −1.0 (−4.0 to 2.3) | 0.386 |
| DPA, steps-day | |||
| Before | 5913 (3829–8395) | 5795 (2470–7670) | 0.537 |
| After | 5876 (3429–8882) | 6786 (2842–8538) | 0.890 |
| Change from baseline | 7.0 (−1380–1270) | −47.5 (−466 to 1482) | 0.555 |
Data are presented as median (Q1–Q3).
CAT, COPD Assessment Test; DPA, Daily Physical Activity.
The response of patients with ferritin<30ng/mL to iron therapy was further analyzed and described in Table 5.
Impact of ferric carboxymaltose treatment in patients with ferritin<30ng/mL.
| Ferritin<30ng/mL (N=10) | Ferritin>30ng/mL (N=34) | ||
|---|---|---|---|
| Endurance time, seconds | |||
| Before | 273.5 (255.0–310.0) | 286.0 (242.5–359.5) | 0.730 |
| After | 553.0 (456.0–649.0) | 406.0 (269.5–497.0) | 0.003 |
| Change from baseline | 297.0 (193.3–335.3) | 53.0 (−5.0 to 216.5) | 0.001 |
| CAT questionnaire | |||
| Before | 13.5 (10.8–21.3) | 15.0 (8.0–19.5) | 0.880 |
| After | 5.0 (4.0–11.5) | 12.0 (6.0–18.5) | 0.096 |
| Change from baseline | −7.5 (−16.5 to 0.8) | −2.0 (−5.0 to 2.0) | 0.075 |
| DPA, steps-day | |||
| Before | 6038 (4570–6752) | 5913 (3404–8881) | 0.936 |
| After | 6358 (3695–12,033) | 5851 (3016–8882) | 0.409 |
| Change from baseline | 291 (−1151 to 4804) | − 651 (−1380 to 1167) | 0.211 |
Data are presented as median (Q1–Q3).
CAT, COPD Assessment Test; DPA, Daily Physical Activity.
Close to a quarter (22.7%) of patients receiving treatment presented less than 30ng/mL of ferritin. These patients presented a more favourable response, as all of them patients achieved the primary endpoint versus only 38.2% in those with normal ferritin levels [p=0.001, RR=1.77 (95% CI, 1.24–2.53)]. Overall, these patients improved their endurance time by 101.3% vs. 17.6% in the ferritin>30ng/mL group (p=0.001). Furthermore, a trend towards an improvement in the CAT questionnaire was also noticed.
Safety dataNo differences were reported between both study groups concerning safety. More specifically, no injection-site pain, discoloration or hypersensitivity reactions were observed after 4 weeks from the treatment, and blood pressure was within normal values throughout the entire course in all patients. Moreover, 6.3% of patients having received treatment presented COPD exacerbation vs. 8.3% in the placebo group (p=0.656; RR: 0.79 [95% CI 0.26–2.46]; Risk reduction: −2.33% [95% CI −15.20–10.53]; number needed to harm 43 patients) but none of these patients required hospital admission or died. Oral antibiotics were prescribed in two cases and oral corticosteroids were given in the rest. Exacerbation was not considered to be related to the study drug and was defined as grade 2 side effect.
Only one patient did not tolerate the treatment with intravenous iron (2.3%, Risk Reduction: 2.3% [95% CI −2.13–6.68]; number needed to harm 46 patients). This adverse event was considered as grade 1 (mild effect; intervention not indicated) and treatment infusion was continued without further events.
DiscussionTo the best of our knowledge, this is the largest study assessing the impact of the intravenous iron supplementation on exercise tolerance in COPD patients with ID with or without mild anaemia. The main finding of this study was that the treatment with intravenous ferric carboxymaltose was associated with a larger improvement in the endurance time compared to placebo, even in patients without mild anaemia. The results of our study confirm our previous hypothesis and fit with those found in other chronic diseases. Based on our predefined subgroup analysis, we also found that patients with a ferritin value<30ng/mL performed strikingly better. However, this is only a hypothesis generating data. Finally, iron treatment was well tolerated without significant safety issues.
ID is estimated to affect two billion people worldwide23 being the leading cause of anaemia.24 Moreover, ID appears to be responsible for 0.8% of all deaths and 1.3% of all disability-adjusted life years.24 In COPD patients in particular, anaemia and ID are quite common. However, the former is neither sought for or, if found, not treated.25 Furthermore, NAID is observed in almost 40–50% of patients.8,26 The present study shows a NAID prevalence of 40% in a screening of 200 patients. However, although ID and NAID are associated with reduced exercise tolerance and QoL,16 current guidelines of COPD2 did not recognize these abnormalities as common and clinically relevant comorbidities. In contrast, the 2016 European Society of Cardiology guidelines for the diagnosis and treatment of acute and CHF do recommend ferric carboxymaltose for the treatment of ID to alleviate heart failure symptoms and improve exercise capacity and QoL.27,28
To date, studies on ID and resultant decreased exercise performance have been performed predominantly in animal models, while data regarding the relationship between ID and skeletal muscle dysfunction in ID anaemic human subjects are limited and inconsistent.9 Furthermore, the benefits of iron replacement have been found in cardiopulmonary diseases other than COPD such as CHF and PAH with ID, with or without anaemia.11,13,15,29–32 In the largest randomized controlled clinical trial including 459 patients with CHF, Anker et al.11 showed that treatment with intravenous ferric carboxymaltose improved symptomatology, functional capacity and QoL with an acceptable safety profile. Similarly, this treatment can also improve clinical outcomes in PAH patients with ID.33 Moreover, Viethen et al.32 studied the treatment with a single dose of ≤1000mg of ferric carboxymaltose in 20 patients with pulmonary hypertension and ID, reporting a significant improvement not only in iron status but in the 6MWT distance and QoL.
While the effort limitation mechanisms in iron deficiency anaemia may also include oxygen transport, when there is NAID, it might be the result of diminished oxygen utilization in the muscle cells due to impaired synthesis or function of different iron-containing enzymes.6 In fact, experimental data from in vitro and animal studies indicate that skeletal muscle network of pathways for energy production is hampered by ID at different points in the distinct metabolic routes.9 ID affects mitochondrial morphology (such as a decrease in density of cristae in the mitochondrial inner membrane) and dramatically impairs the process of mitochondrial oxidative phosphorylation.9 All these effects may add to the general decrease in oxidative metabolism efficiency.9 This is one of the hypotheses by which NAID may have a negative impact on aerobic capacity16 and can produce fatigue and exercise intolerance34 in patients with demonstrated peripheral muscle dysfunction.9,35 On the other hand, it should be mentioned that peripheral muscle strength did not improve following treatment with iron in any of the study groups. These results suggest that factors other than muscle function are major contributors to general exercise tolerance in severe COPD patients, as has been shown in previous reports.36–39 As such, it has been demonstrated a mismatch between ventilatory capacity and the patients’ demand, an imbalance between energy requirements and supply to the working respiratory and peripheral muscles, and other factors that contribute to muscle dysfunction.37 The effects of iron supplementation may go beyond those of skeletal muscle performance. Organs such as the vascular endothelium, myocardium, and the lungs and airways may have also contributed to the improvement in ET seen in the patients who received iron replacement.36–39 Another interesting possibility is the decrease in myoglobin reserves in the muscle, which would affect its bioenergetics. As Hb, myoglobin is an iron- and -oxygen binding protein. Its function is not completely elucidated but it is believed that it also participates in oxygen storage within the muscle increasing the availability of this gas for aerobic purposes.40 On the other hand, myoglobin as Hb are scavengers of reactive oxygen species.41 This would limit oxidative stress, one of the mechanisms involved in muscle dysfunction repeatedly observed in COPD.42
Considering all this evidence, it is not surprising that in the aforementioned randomized clinical trial conducted in patients with CHF, the treatment with ferric carboxymaltose was beneficial to both patients with anaemia and those without anaemia.11 Our study clearly shows similar results in moderate to severe COPD patients either with mild anaemia or NAID. This suggests that the presence of ID is a valid independent therapeutic target in different chronic diseases. We hypothesize that iron availability in skeletal muscles could have improved their oxidative capacity,14 increasing the time to the anaerobic threshold during submaximal exercise.31 The only previous clinical trial analysing the impact of intravenous iron in COPD was recently published by Santer et al.17 Their primary endpoint (oxygen saturation) was not met although exercise capacity (assessed by the 6MWT) improved. However, this might be explained by the fact that patients’ baseline oxygen saturation was only marginally (<1%) lower than that of age-matched general population.17 The exact mechanisms leading to anaemia and ID in COPD are complex and remain not fully understood. Moreover, iron metabolism in patients with this and other chronic illness merits a more detailed investigation to better unravel the reasons why the correction of ID can result in symptomatic improvements even in the absence of changes in Hb.
Interestingly, we have also observed that the improvement in exercise capacity was especially important in those patients with ferritin<30mg/dL.24,43 This is probably related to the main functions of this protein, which are iron storage, transportation, and delivery to the tissues. However, our finding deserves further studies to completely understand the mechanisms of such a higher improvement.
Our study achieves the primary endpoint showing a 3-fold possibility of a 33% improvement in the endurance time with iron treatment when compared to placebo administration. Although this specific improvement (33%) was defined by the European Respiratory Society task force for nonpharmacological interventions,44 we strongly believe that it should be also valid for pharmacological treatments. On the other hand, the election of the endurance time during CWRET as the primary endpoint was based on the previous use of this variable as the outcome measure in other studies, including those using ferric carboxymaltose.19,45
Regarding the secondary endpoints of the present study, we also observed an improvement in CAT questionnaire in those patients who received ferric carboxymaltose. However, DPA did not change. Our hypothesis for this latter result is that all patients were sedentary, and changes in this dimension normally also involve a behavioural intervention.46
Study critiqueVitamin B12 and folic acid play an important role in the synthesis of haemoglobin and are essential in the homocysteine, mitochondrial, muscle metabolism and haematopoiesis.47 This is a relevant issue as previous results showed that up to 34.4% of COPD patients may exhibit vitamin B12 deficiency.47 However, in our cohort, both patients with NAID and mild anaemia presented mean corpuscular volume within normal ranges (<100fl), thus ruling out the possibility that macrocytic anaemia due to vitamin B12 deficiency may have played any role in the reported findings.
A potential limitation may be related to the lack of maximal inspiratory pressure (PImax) measurements in the study patients. Despite the potential interest of this aspect, the study aimed to assess whether treatment with iron may improve exercise tolerance in COPD patients and eventually DPA. Thus, the focus was rather on peripheral limb muscles than on respiratory muscles. Furthermore, PImax does not necessarily predict the patients’ performance on the cardiopulmonary exercise test, which may limit its usefulness in this setting.48 On the other hand, inspiratory muscle weakness was independently associated with skeletal muscle mass in men with systolic chronic heart failure and ID.49
It should also be mentioned that mechanisms other than the potential contribution of peripheral muscle dysfunction to exercise tolerance may exist. Alterations in vascular lung homeostasis, namely the reversal of haemodynamic and pulmonary vascular remodelling by iron replacement may have also influenced the results, as also shown to occur in a mouse model of NAID.50 Moreover, intracellular iron deficiency within the pulmonary arterial smooth muscle cells also altered lung vascular function, as demonstrated in another mouse model.51 Despite the relevance of these findings, the current clinical trial was not designed to target these aspects in the cohort of COPD patients. Future studies should be specifically designed to address these relevant questions.
Other limitations should be considered such as the fact that only 4 patients in the subgroup with mild anaemia received carboxymaltose. Although the increase in haemoglobin levels could be involved in the improvement of exercise capacity, the small numbers preclude the analysis of the impact of haemoglobin on the study outcomes. Finally, it should be mentioned that this was a single-blind clinical trial and that the trial was conducted only in one centre, which may question the potential extrapolation of the study results to the general population of COPD patients. However, the current results will contribute to the design of multicentric randomized clinical trials soon.
ConclusionsThe FACE study has shown that intravenous iron carboxymaltose therapy is well tolerated and associated with improvements in exercise tolerance and symptoms in patients with COPD and iron deficiency, with or without anaemia. These benefits were even more evident in patients with low ferritin levels. The assessment of the iron profile and correction of iron status may therefore become part of the follow-up and management of COPD patients. Further studies will answer several unsolved questions such as the potential underlying molecular mechanisms, the long-term benefits of the treatment, and the needs for more infusions.
FundingThis study has been funded by Instituto de Salud Carlos-III (ISCIII), contract grants FIS 17/00649 and 18/00075 [FEDER]), BA 17/00025. CIBERES, ESF02/2017; Spanish Respiratory Society (SEPAR) 409/2017; Catalan Foundation of Pulmonology (FUCAP) 2017; and an unrestricted grant from Vifor Pharma 2018. Vifor Pharma did not have any role in the study or statistical analyses.
Conflict of interestNone.
The authors would like to thank Diana Badenes Bonet, Ana Balañá Corberó, Concepción Ballano Castro, Roberto Chalela Rengifo, Mercè Espona, Jose Gregorio González García, Laura Gutiérrez Martín, and Ignacio Vicente for their assistance in collecting data for this study.