Apalutamide is an androgen receptor signaling inhibitor (ARSi), indicated as standard of care in 2019 in the treatment of metastatic castration-sensitive prostate cancer (mCSPC) together with androgen deprivation therapy (ADT).1 The incidence of interstitial lung disease (ILD) associated with apalutamide is very uncommon with few cases of this side effect reported on the literature.2–4 ILD can become serious so clinicians should be alerted to this potential adverse effect and know the clinical management in this situation. Here, we present two cases of apalutamide-induced interstitial lung disease who were successfully treated with high-dose corticosteroids.
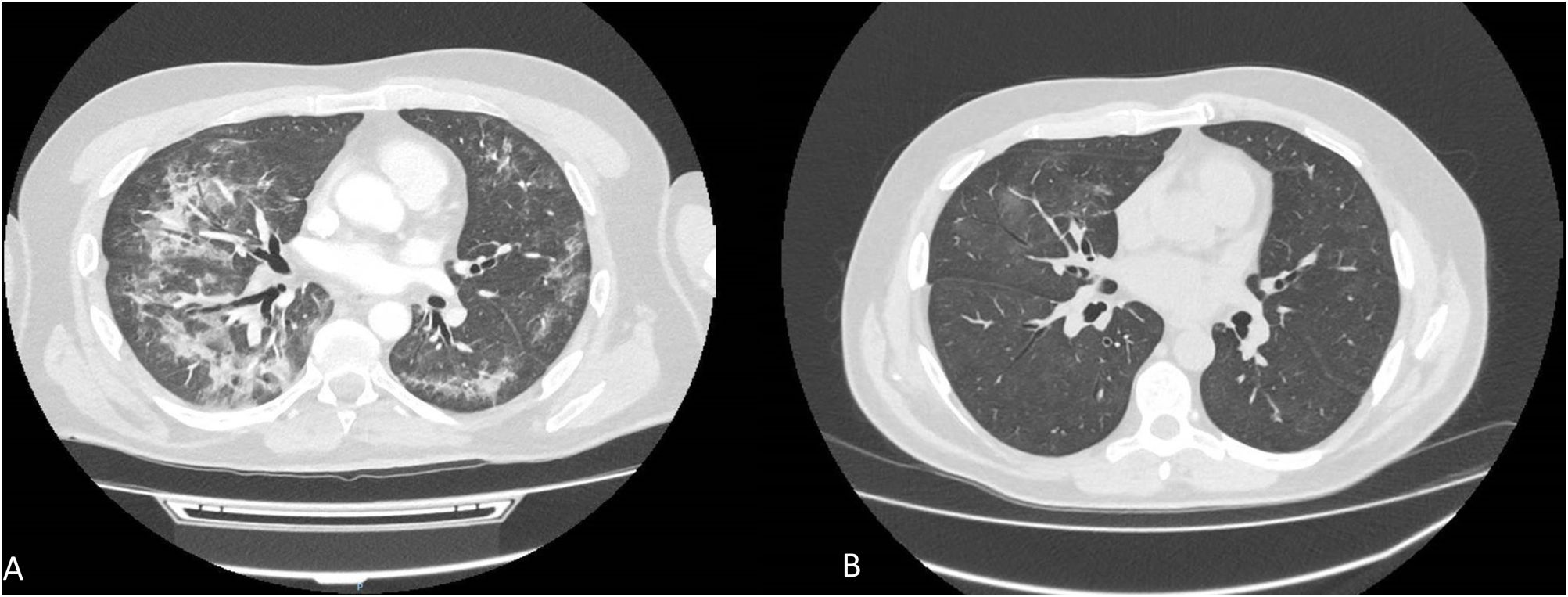
The first case refers to a 55-year-old man without medical history of interest diagnosed of metachronous low volume mCSPC. He initiated Triptorelin 22.5mg and a month later initiated apalutamide. The dose of apalutamide was reduced from 240mg to 180mg per day after two months due to the appearance of grade III skin rash. One month later, the patient experienced cough and progressive respiratory distress. On auscultation, fine crackles were heard in both lungs. Chest CT showed subpleural parenchymal bands, ground-glass opacities and bilateral consolidations (Fig. 1A), without alterations in previous imaging test. Based on these findings and on the absence of any other suspected drug, the diagnosis of apalutamide-induced ILD was made, so apalutamide was discontinued and endovenous metilprednisolone 1mg/kg/day was started. After five days, the treatment was switched to oral deflazacort 80mg/day for seven days, progressively reducing 20mg per week until discontinued. The patient improved his respiratory status after 15 days of corticosteroids treatment. Chest-CT at 3 months after discontinuing apalutamide, showed a significant improvement with resolution of previously described pulmonary signs (Fig. 1B).
Patient 1. (A) CT scan shows subpleural parenchymal bands, ground-glass opacities and bilateral consolidations in both lung fields at 3 months after started apalutamide. (B) CT scan at 3 months after discontinuing apalutamide, showed a significant improvement with resolution of previously described pulmonary signs.
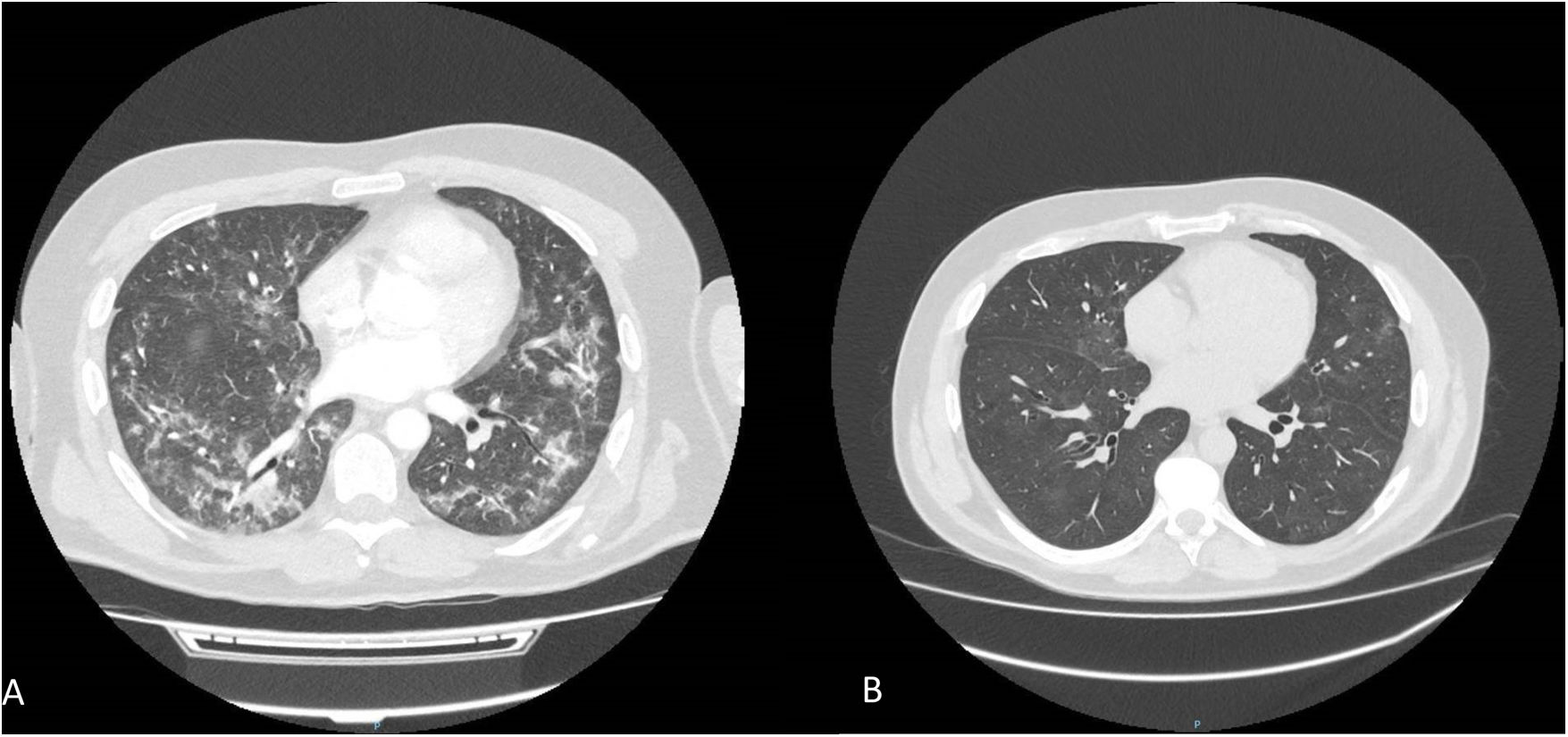
The second case refers to a 73-year-old man without medical history of interest diagnosed “de novo” mCSPC and treated with radiotherapy to the prostate as well as leuprorelin 45mg and apalutamide (240mg/day). Six months after initiation of apalutamide he described an evolution of two months of cough, mucous expectoration and weight loss, the patient was admitted to pneumology due to clinical worsening and acute respiratory failure. Chest-CT showed bilateral patchy areas of ground-glass opacities with bronchiectasis (Fig. 2A), without alterations in previous imaging test. Bronchoscopy with cytology only appeared inflammatory cellularity (9% granulocytic polynuclears, 1% eosinophils, 5% monocytes, 27% alveolar macrophages, 52% lymphocytes with CD4/CD8 ratio of 0.25). Infectious etiology was ruled out through PCR, blood cultures and BAS citology. He was diagnosed of apalutamide-induced ILD, so he started endovenous metilprednisolone 1mg/kg/day and switched to oral prednisone 60mg/day for fifteen days, progressively reducing 7.5mg per week until discontinued with rapid resolution of the dyspnea. CT reassessment two months after showed practical resolution of the pulmonary signs (Fig. 2B).
Currently both patients are undergoing follow-up observation with ADT treatment without progression and no respiratory symptoms. Apalutamide was no re-administered in both cases.
Drug-induced ILD is a serious condition that occurs in 3–5% of all cases of ILD ad it is associated with antineoplastic drugs like bleomycin, targeted drugs, immune checkpoint inhibitors or antiandrogens (bicalutamide, flutamide, goserelin, degarelix and apalutamide) in which it tends to occur during first year after starting treatment.4,5 In our cases, it manifested at 3 and 6 months respectively. Although cases of ILD have been described in the literature with gonadotropin releasing hormone agonist,4 in our reported cases we did not find a causal relationship with ILD since the patients continued treatment with ADT without recurrence of symptoms and with radiological improvement. The occurrence of ILD due to apalutamide has been described in adverse effects databases,4,5 but there are only a few cases reported in Asian patients.2,3 To the best of our knowledge these are the first two cases of apalutamide-induced ILD reported in European patients.
In conclusion, apalutamide-induced ILD is a rare side effect that must keep in mind due to its potential severity, so lung function should be monitored in patients treated with ARSi, especially during first year of therapy.
Authors’ contributionsRocío del Castillo-Acuña: Writing-review & editing. Ana Serradilla: Writing-review & editing. Fernando López-Campos: Writing-review & editing. Supervision. Felipe Couñago: Writing-review & editing. Supervision.
Conflict of interestsThe authors state that they have no conflict of interests.