There is no specific health-related quality of life (HRQL) questionnaire that has been validated in Spanish for its use in patients with sleep apnea-hypopnea syndrome (SAHS). The objective of the present study was to validate the Spanish version of the Quebec Sleep Questionnaire (QSQ).
Patients and methodsA multi-center study including a group of patients with SAHS (AHI≥5) referred to the Sleep Unit. All patients completed the following questionnaires: SF-36, FOSQ, QSQ and Epworth scale. Internal consistency, construct validity, concurrent validity, predictive validity, repeatability and responsiveness to change of the QSQ (32 items in five domains: daytime sleepiness, diurnal symptoms, nocturnal symptoms, emotions, and social interactions) were assessed.
ResultsOne hundred and twenty-one patients were included in the study (mean age: 57±13; mean Epworth: 9±4; mean body mass index (BMI): 28±3kgm−2 and mean AHI: 36±20h−1). The factorial analysis showed a construct of five factors with similar distribution to the original questionnaire domains. Internal consistency (Cranach's alpha between 0.78 and 0.93 for the different domains), concurrent validity (compared to SF-36, Epworth scale and FOSQ), predictive validity of SAHS severity and test–retest reliability were appropriate. The test showed good responsiveness to change in diurnal (P=.003) and nocturnal symptoms domains (P=.02).
ConclusionsThe Spanish version of the QSQ is a valid HRQL measure with appropriate psychometric properties for use in patients with SAHS and is responsive to change in symptoms domains.
No existe ningún cuestionario específico de calidad de vida validado en castellano para su uso en pacientes con síndrome de apneas-hipopneas durante el sueño (SAHS). El objetivo del presente estudio fue validar la versión castellana del Quebec Sleep Questionnaire (QSQ).
Material y métodosEstudio multicéntrico en un grupo de pacientes diagnosticados de SAHS (IAH≥5) enviados a las Unidades de Sueño. En todos los pacientes se administraron los cuestionarios: SF-36, FOSQ, QSQ y test de Epworth. Se evaluaron las propiedades psicométricas (consistencia interna, validez de constructo, validez concurrente, validez predictora, fiabilidad test-retest y sensibilidad al cambio) del cuestionario QSQ (32 ítems en cinco dominios: somnolencia diurna, síntomas diurnos, síntomas nocturnos, emociones e interacciones sociales).
ResultadosSe incluyeron 121 pacientes (edad media: 57±13 años; Epworth: 9±4; IMC 28±3kg·m−2 e IAH 36±20h−1). El análisis factorial mostró un constructo de cinco factores, distribuidos de manera similar a los dominios del cuestionario original. Tanto la consistencia interna (alfa de Cronbach entre 0,78 y 0,93 para los distintos dominios), la validez concurrente con respecto al SF-36, Epworth y FOSQ, la validez predictora de gravedad del SAHS, como la fiabilidad test-retest fueron adecuadas. El QSQ mostró una buena sensibilidad al cambio en los dominios relativos a los síntomas diurnos (p=0,003) y nocturnos (p=0,02).
ConclusionesLa versión española del QSQ presenta unas características psicométricas adecuadas para su utilización en pacientes con SAHS, así como una sensibilidad al cambio significativa en los dominios de síntomas.
With the current state of evidence, there is no doubt that sleep apnea-hypopnea syndrome (SAHS) is today a public health problem due to the high prevalence that it reaches in the general population (estimated at 4% of men and 2% of women1) as well as its negative effects on health, especially those related to cardiovascular morbidity and mortality, neuropsychiatric alterations and the loss of quality of life of the individuals with SAHS.2–4
One of the less frequently evaluated aspects in the literature is the impact that SAHS has on health-related quality of life (HRQL). To evaluate this impact, validated questionnaires are required, especially for the specifics of the disease in question. Such surveys provide great sensitivity for detecting the changes produced after therapeutic alterations and are able to discern the modifications in HRQL caused by respiratory disorders during sleep or of other chronic diseases.5 The most commonly used and evaluated generic questionnaire is the SF-36, which has been validated in Spanish6 and has been demonstrated to be a very adequate tool for its use in research and clinical practice.7,8 Among the specific questionnaires for evaluating the quality of life of patients with SAHS are the Sleep Apnea Quality of Life Index (SAQLI),9 which has been translated, adapted and validated in Chinese and Lithuanian.10,11 The Quebec Sleep Questionnaire (QSQ) was created by Lacasse et al. in French, their native tongue, and later translated to English for its use in patients with SAHS, demonstrating some excellent psychometric qualities in the English-language version.12,13 Spanish, with 329 million speakers, is currently the second most widely spoken language after Chinese (1.213 billion), followed by English (328 million).14 Although the QSQ has been successfully translated and adapted to Spanish,15 it has not been demonstrated whether this version has the psychometric qualities necessary for its use in patients with SAHS. Given that until now there has been no specific questionnaire for SAHS validated in Spanish in the literature, the objective of our study was to analyze the reliability and validity of the Spanish version of the Quebec Sleep Questionnaire HRQL survey in SAHS patients.
Material and methodsStudy SampleOurs is a multi-center, prospective study that lasted for one year. One hundred and sixty consecutive patients over the age of 18 were initially recruited from 3 centers of the Community of Valencia that have extensive clinical and research experience in sleep pathologies. The patients had been sent to the respective specialized outpatient consultations due to clinical suspicion of SAHS. Excluded from the study were all those patients who presented significant unstable comorbidities that could influence the study conclusions, patients with important cognitive disorders and those who refused to form part of the study or were not able to complete the questionnaires.
Data CollectionIn accordance with the study protocol, data were collected from all the patients for the following variables: age, sex, SAHS symptoms, the Spanish version of the Epworth test,16 sleep study variables and those referring to the quality-of-life questionnaires used (FOSQ [Functional Outcomes in Sleep Questionnaire],17–19 SF-36 [Medical Outcome Survey-Short Form 36] and the Spanish version of the QSQ [Appendix 1]). Prior to the study, all the patients signed an informed consent form and the study was approved by the ethics committees of the participating centers.
Sleep StudyThe SAHS diagnosis was done using Somnea® or Embletta® hospital respiratory polygraphy (RP) or instead with conventional polysomnography (PSG) (Sleeplab, Jaeger®) and all the tracings were analyzed manually. Treatment with nasal continuous positive airway pressure (CPAP) was considered in patients who presented an apnea/hypopnea index (AHI) ≥5 together with symptoms related with SAHS and in patients with AHI ≥30. Pressure adjustments were determined with either complete PSG or by means of a validated hospital auto-CPAP system (ResMed S8 AutoSet™ II Auto CPAP). Proper CPAP compliance was considered a use of ≥4h per day, 70% of the days of the week. In all cases, national guidelines were followed.1
Study ProtocolAt the initial office visit, the baseline data of all the patients were collected, including general variables and SAHS symptom variables, sleep study data and the different quality of life test scores (FOSQ, SF-36 and the Spanish version of the QSQ) that were either self-administered or assisted by an interviewer. At a later visit one week afterwards, a computer program with randomized numbers chose 14 patients at random who once again answered the QSQ for the analysis of repeatability. Last of all, at a final visit that was at least 10 weeks after the previous visit, 31 patients were randomly selected from the CPAP group that had criteria for good tolerance and compliance in order to study the sensitivity to change of the questionnaire.
Statistical AnalysisThe QSQ, FOSQ and SF-36 were scored in accordance with the instructions of the authors of the original scales. All the statistical analyses were done with SPSS software for Windows, version 11.5 (Chicago, IL, USA). A descriptive analysis was developed both of the clinical parameters as well as of the quality of life parameters, expressed as means±standard deviation in the case of quantitative variables and as absolute value and percentage of the total in the qualitative variables. For the comparison of two means, the Student's t-test was used, while for the comparison of more than two means a variable analysis (ANOVA) was used with the Bonferroni correction or the corresponding non-parametric tests if the variables did not have normal distribution (normality was verified with the Kolmogorov–Smirnov test). In order to compare two dichotomic variables, the χ2-test was used.
In order to analyze the internal consistency, the Cronbach alpha coefficient was calculated for each of the five domains of the questionnaire. According to Nunnany,20 an alpha value higher than 0.7 is considered sufficient in order to use the questionnaire in the comparison between patient groups. The construct validity was analyzed with a factorial analysis of main components whose applicability was verified by means of Bartlett's test of sphericity and the KMO test (acceptable with values above 0.5). Each item was included in a specific factor if there was a minimal degree of saturation of 0.4 and an eigenvalue greater than 1. The number of factors was determined without structure restrictions according to the result of the scree test and the analysis of the sedimentation chart. In order to analyze the concurrent validity, the Pearson or Spearman correlation values were used, depending on the normality of the distribution of the variables, the different domains of the QSQ with the Epworth test and the domains of the SF-36 and FOSQ questionnaires that referred to similar characteristics. The predictive validity was analyzed comparing the patient groups with severe or non-severe SAHS according to an AHI cut-point of 30 using a Student's t-test for independent means. The test-retest reliability (repeatability) was evaluated with the intraclass correlation coefficient. ICC values above 0.71 were considered to have a good agreement, while values between 0.51 and 0.70 were considered moderate.21 Lastly, sensitivity to change was analyzed at least 10 weeks after the effective treatment with CPAP, comparing the values of all the domains of the Quebec questionnaire before and after the treatment using a Student's t-test for repeat means. A P<.05 was considered statistically significant.
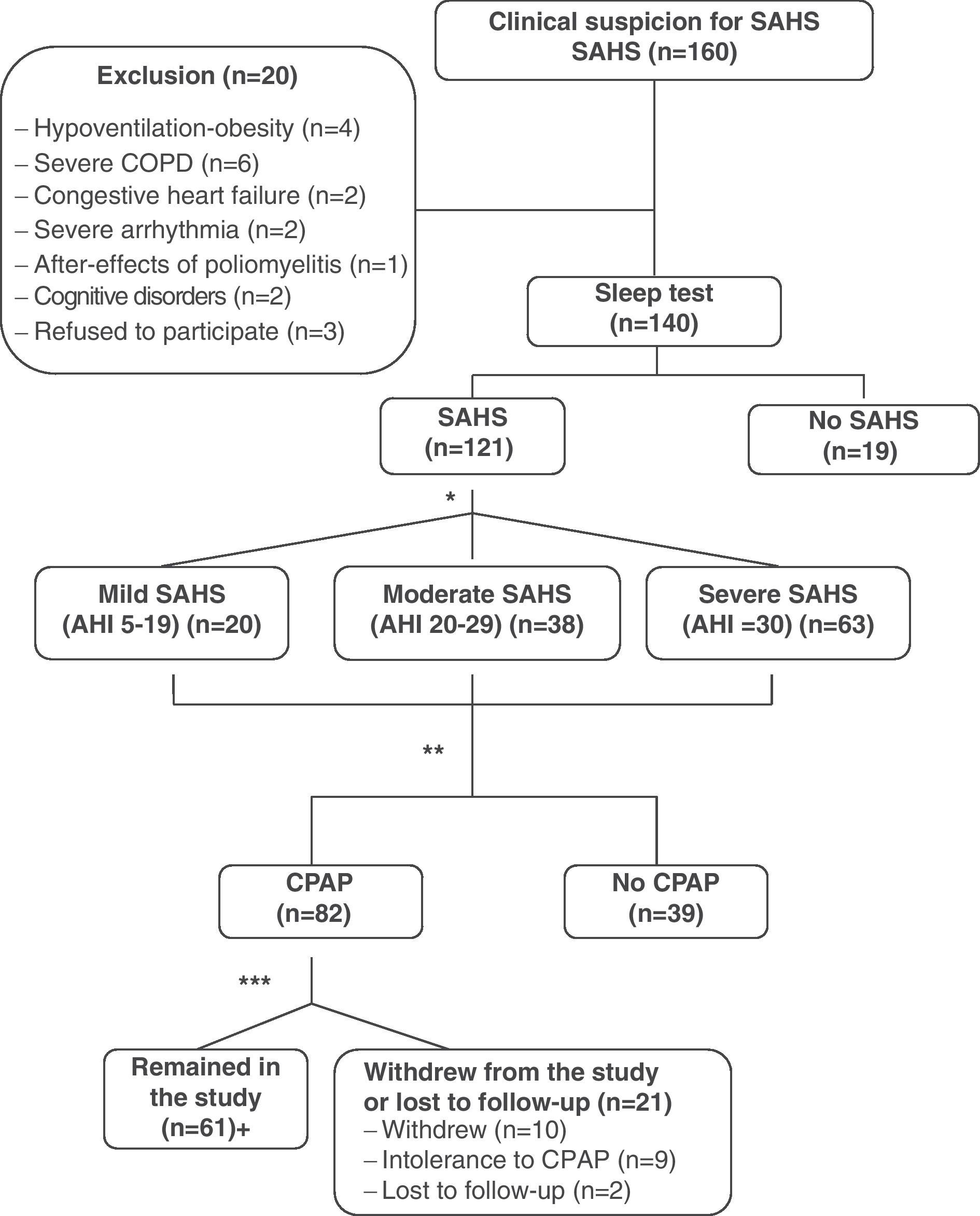
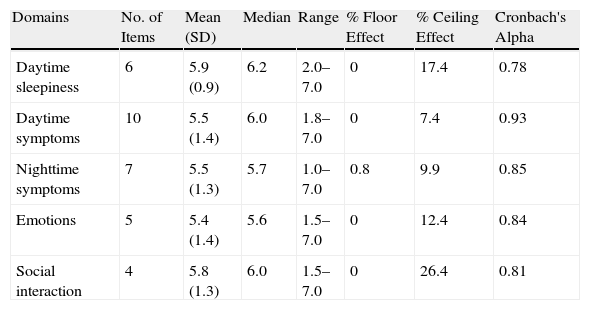
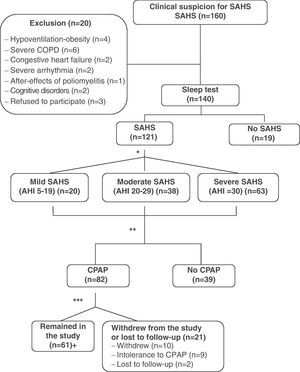
ResultsDuring the study period, 160 consecutive patients were remitted to the specialized outpatient sleep consultation due to clinical suspicion of SAHS. Twenty patients were excluded, of which 15 presented significant unstable comorbidities (4 hypoventilation-obesity, 6 severe COPD, 2 congestive heart failure, 2 severe arrhythmia, 1 after-effects of poliomyelitis); 2 patients were excluded due to important cognitive disorders and 3 refused to take part in the study. Out of the 140 patients who were finally included, 121 (86.4%) were diagnosed with SAHS, whose data were used for the analysis of internal consistency and validity of the QSQ survey. The mean age of these patients was 57±13, with an Epworth scale of 9±4. The mean AHI was 36±20h−1 (range: 5–89) and the body mass index (BMI) was 28.1±3.2kgm−2. Of all the patients studied, 82 received treatment with CPAP, following SEPAR guidelines. Sixty-one patients tolerated and complied with a minimum treatment of 4h daily. Out of the remaining 21 patients treated with CPAP, 10 abandoned the study, 9 did not tolerate the treatment and 2 patients were lost to follow-up (Fig. 1). We found no statistically significant differences between the patients who abandoned the study compared with those who did not abandon for the variables evaluated. Table 1 shows the results for the general description of the QSQ survey (mean, standard deviation, ranges, percentage of patients with ceiling effect and floor effect).
Methodological flowchart of the study. *Following SEPAR guidelines. **Validation of the questionnaire. 14 patients were randomly chosen for the test-retest reliability study. ***A use of ≥4h is considered good tolerance. +31 patients were randomly chosen for the sensitivity-to-change analysis. COPD: chronic obstructive pulmonary disease; SAHS: sleep apnea-hypopnea syndrome; CPAP; continuous positive airway pressure.
General Description and Internal Consistency of the Results of the Quebec Sleep Questionnaire (QSQ).
| Domains | No. of Items | Mean (SD) | Median | Range | % Floor Effect | % Ceiling Effect | Cronbach's Alpha |
| Daytime sleepiness | 6 | 5.9 (0.9) | 6.2 | 2.0–7.0 | 0 | 17.4 | 0.78 |
| Daytime symptoms | 10 | 5.5 (1.4) | 6.0 | 1.8–7.0 | 0 | 7.4 | 0.93 |
| Nighttime symptoms | 7 | 5.5 (1.3) | 5.7 | 1.0–7.0 | 0.8 | 9.9 | 0.85 |
| Emotions | 5 | 5.4 (1.4) | 5.6 | 1.5–7.0 | 0 | 12.4 | 0.84 |
| Social interaction | 4 | 5.8 (1.3) | 6.0 | 1.5–7.0 | 0 | 26.4 | 0.81 |
SD: standard deviation; % Ceiling effect: percentage of patients who reached the maximum score on each scale or on the total score of the questionnaire; % Floor effect: number of patients who reached the lowest score on each scale or on the total score of the questionnaire.
The Spearman's correlation coefficient between the different items of the questionnaire varied in each of the domains in the following manner: for daytime sleepiness, it fluctuated between 0.54 and 0.79, and was always significant; in daytime symptoms, the correlations were between 0.14 and 0.86, most of them significant; in nighttime symptoms, the correlations were between 0.04 and 0.79; the emotions varied in a range between 0.09 and 0.78; in social interactions, the correlations fluctuated between 0.17 and 0.86. In each of the domains, the majority of the correlations were significant, and nearly significant in the case of the emotions and social interaction domains. The Cronbach alpha for each of the domains was: 0.78 for daytime sleepiness, 0.93 for daytime symptoms, 0.85 for nighttime symptoms, 0.84 for emotions and 0.81 for social interactions (Table 1).
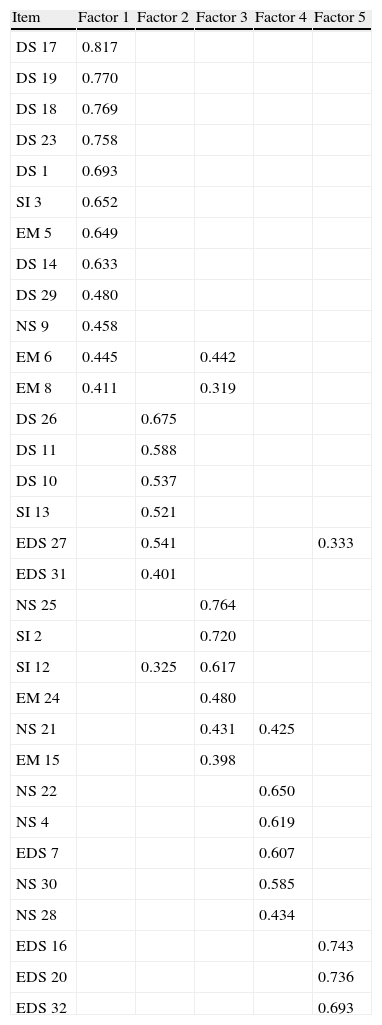
Construct ValidityBartlett's test of sphericity was significant (P<.0001) and the KMO test was 0.834. All this enabled a factorial analysis to be applied to the matrix of correlations. Using the scree test and the sediment chart analysis, 5 factors were determined that explained 57.2% of the variance. The rotation used, given the correlation among these factors, was orthogonal (varimax) (Table 2). All the items were introduced in the analysis. The first factor was made up of 12 items with an explained variance of 19.2%. The structure was similar to the scale of daytime symptoms of the original questionnaire and grouped 7 of the 10 items of this scale. It also included 4 items of the emotions domain. The second and third factors explained 10% more of the variance each, without being clearly related with a specific domain. The fourth factor is made up of 6 items that explains another 9.6% of the variance, with a structure similar to the domain of nighttime symptoms, grouping 4 of the 7 items of this domain. Lastly, the fifth factor, made up of 3 items, all of them with significant saturation, explains 7.7% more of the variance and is completely made up of items corresponding to the daytime sleepiness domain.
Matrix of the Five Factors Extracted by the Factorial Analysis of Main Components With Varimax Rotation (Only Those Items With Saturations Higher Than 0.30 Are Shown).
| Item | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 |
| DS 17 | 0.817 | ||||
| DS 19 | 0.770 | ||||
| DS 18 | 0.769 | ||||
| DS 23 | 0.758 | ||||
| DS 1 | 0.693 | ||||
| SI 3 | 0.652 | ||||
| EM 5 | 0.649 | ||||
| DS 14 | 0.633 | ||||
| DS 29 | 0.480 | ||||
| NS 9 | 0.458 | ||||
| EM 6 | 0.445 | 0.442 | |||
| EM 8 | 0.411 | 0.319 | |||
| DS 26 | 0.675 | ||||
| DS 11 | 0.588 | ||||
| DS 10 | 0.537 | ||||
| SI 13 | 0.521 | ||||
| EDS 27 | 0.541 | 0.333 | |||
| EDS 31 | 0.401 | ||||
| NS 25 | 0.764 | ||||
| SI 2 | 0.720 | ||||
| SI 12 | 0.325 | 0.617 | |||
| EM 24 | 0.480 | ||||
| NS 21 | 0.431 | 0.425 | |||
| EM 15 | 0.398 | ||||
| NS 22 | 0.650 | ||||
| NS 4 | 0.619 | ||||
| EDS 7 | 0.607 | ||||
| NS 30 | 0.585 | ||||
| NS 28 | 0.434 | ||||
| EDS 16 | 0.743 | ||||
| EDS 20 | 0.736 | ||||
| EDS 32 | 0.693 |
EDS: excessive daytime sleepiness; DS: daytime symptoms; NS: nighttime symptoms; EM: emotions; SI: social interaction.
The values in bold correspond with the items included in each factor.
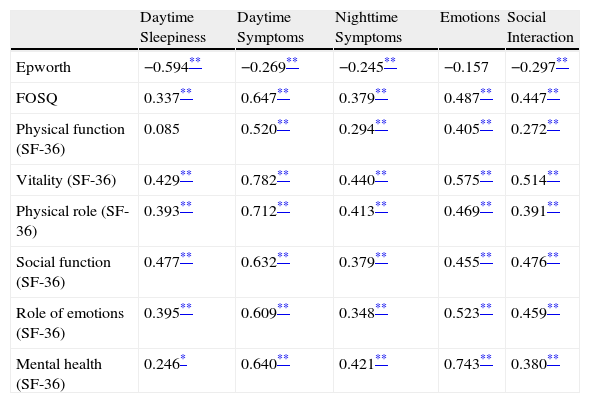
Table 3 shows the correlation (Spearman's coefficient) between the Epworth test and the scores of the domains of the FOSQ and SF-36 compared with the scores of the domains of the QSQ survey that referred to similar characteristics. In general, moderate or high correlations were observed amongst them.
Pearson's Coefficient of the Correlations Between the Quebec Sleep Questionnaire (QSQ) and Related Tools for Measurement.
| Daytime Sleepiness | Daytime Symptoms | Nighttime Symptoms | Emotions | Social Interaction | |
| Epworth | −0.594** | −0.269** | −0.245** | −0.157 | −0.297** |
| FOSQ | 0.337** | 0.647** | 0.379** | 0.487** | 0.447** |
| Physical function (SF-36) | 0.085 | 0.520** | 0.294** | 0.405** | 0.272** |
| Vitality (SF-36) | 0.429** | 0.782** | 0.440** | 0.575** | 0.514** |
| Physical role (SF-36) | 0.393** | 0.712** | 0.413** | 0.469** | 0.391** |
| Social function (SF-36) | 0.477** | 0.632** | 0.379** | 0.455** | 0.476** |
| Role of emotions (SF-36) | 0.395** | 0.609** | 0.348** | 0.523** | 0.459** |
| Mental health (SF-36) | 0.246* | 0.640** | 0.421** | 0.743** | 0.380** |
The most significant correlations are in bold print.
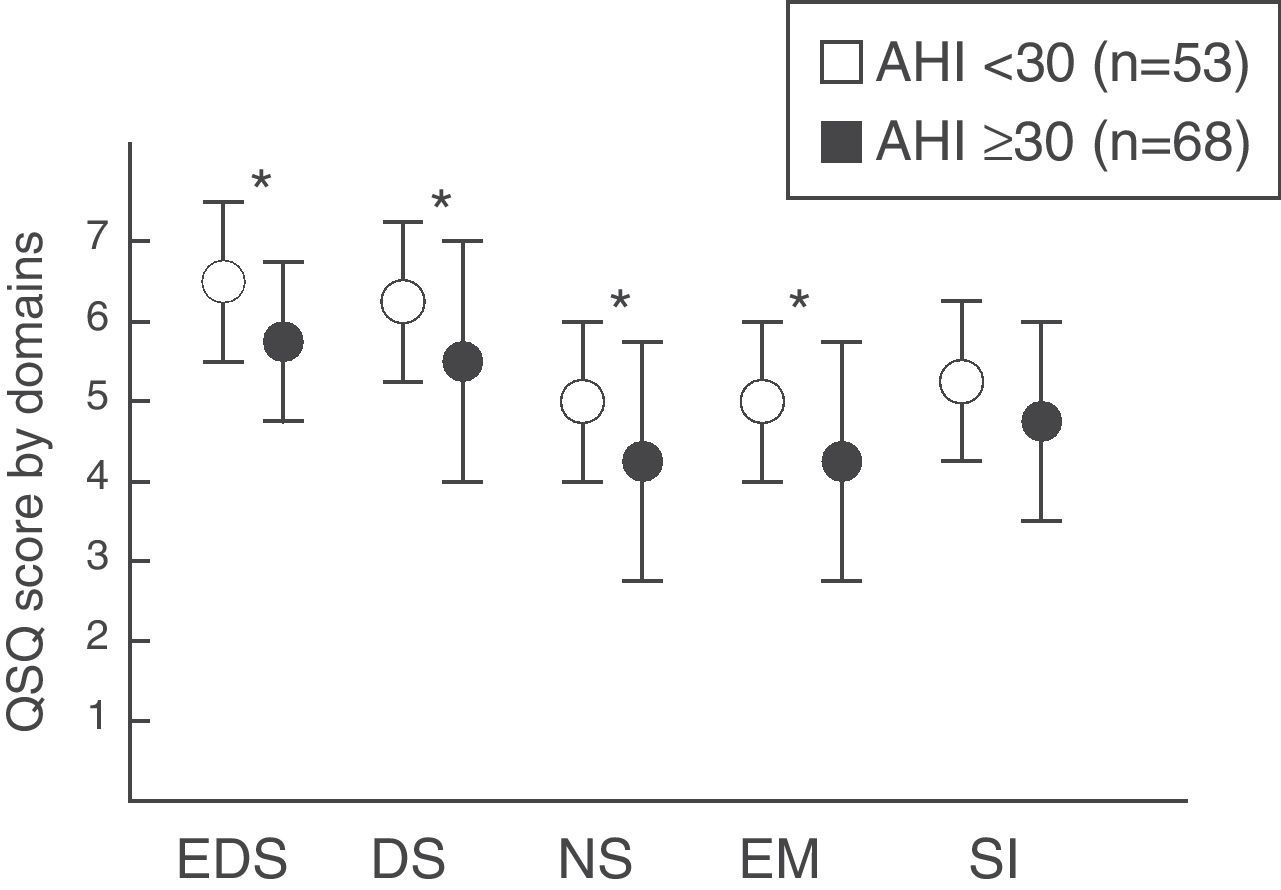
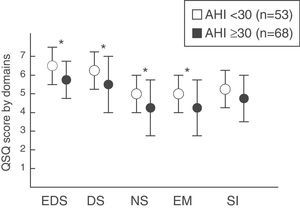
The group of patients with mild–moderate SAHS was compared with those who presented severe SAHS. Statistically significant differences were observed according to the severity in the domains of daytime sleepiness (P=.006), daytime symptoms (P=.012), nighttime symptoms (P=.0001) and emotions (P=.002). However, no differences were found in the social interaction domain (P=.074) (fig. 2).
Quebec Sleep Questionnaire score (mean±standard deviation) according to SAHS severity by groups (mild–moderate vs severe). EDS: excessive daytime sleepiness; DS: daytime symptoms; NS: nighttime symptoms; EM: emotions; SI: social interaction. * P<.05. Values tabulated as mean (standard deviation).
The following intraclass correlation coefficients were obtained for each of the four domains: daytime sleepiness 0.642, daytime symptoms 0.759, nighttime symptoms 0.652, emotions 0.608 and social interaction 0.773.
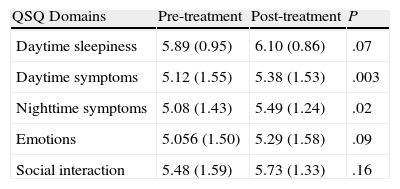
Sensitivity to ChangeTable 4 shows the scores by domains and the differences obtained before and after the treatment. After ten weeks of effective CPAP treatment, a statistically significant change was observed in the domains of daytime symptoms (P=.003) and nighttime symptoms (P=.023), nearly reaching statistical significance in the daytime sleepiness domain (P=.072). No statistically significant differences were found in the emotions or social interaction domains.
Analysis of the Sensitivity to Change. Differences Between the Pre- and Post-CPAP Treatment Values for the Different Domains of the Quebec Sleep Questionnaire (Qsq).
| QSQ Domains | Pre-treatment | Post-treatment | P |
| Daytime sleepiness | 5.89 (0.95) | 6.10 (0.86) | .07 |
| Daytime symptoms | 5.12 (1.55) | 5.38 (1.53) | .003 |
| Nighttime symptoms | 5.08 (1.43) | 5.49 (1.24) | .02 |
| Emotions | 5.056 (1.50) | 5.29 (1.58) | .09 |
| Social interaction | 5.48 (1.59) | 5.73 (1.33) | .16 |
The scores in each of the domains, as well as the differences obtained between pre- and post-treatment, are shown as means and standard deviation (95% confidence interval).
According to our results, the Spanish version of the QSQ presents adequate internal consistency, validity and repeatability for its use in patients with SAHS. Likewise, this questionnaire showed sensitivity to change produced by CPAP treatment for the domains referring to SAHS symptoms, both diurnal as well as nocturnal.
The internal consistency of the tool was high, and the coefficients obtained in each of the domains surpassed 0.7, generally considered acceptable. In the construct validity analysis, we observed a similar structure in three of the five domains, although without finding considerable similarities in the emotion and social interaction domains. Using a factorial analysis, the study showed, after analyzing the sediment chart, that the selection of five factors was similar to the distribution of the original questionnaire in three of them. These factors (1, 4 and 5) explained 36.5% of the variance. In the first factor, referring to daytime symptoms, 7 of the 10 items of this domain were included. The third factor included both items of the emotions domain as well as items of the social interaction domain. The fourth was mainly made up of items corresponding to nighttime symptoms. All the items that were grouped in the fifth factor corresponded with the daytime sleepiness domain. Therefore, there is a good correlation between factors 1, 4 and 5 with the domains of daytime symptoms, nighttime symptoms and daytime sleepiness, respectively, without there being a clear correlation of factors 2 and 3 with the emotions and social interaction domains.
Upon studying the concurrent validity of the test, we observed that there was a strong correlation between the daytime sleepiness domain of the QSQ and the Epworth test, as well as between the daytime symptoms domain of the QSQ and physical function, vitality, social function and the physical role of SF-36. The nighttime symptoms domain likewise presented a moderate correlation with the previously mentioned parameters and with the score of the FOSQ. This does not surprise us because, as we have commented earlier, the items corresponding with these domains are grouped similarly to the original questionnaire, and this has demonstrated similar correlations with the mentioned questionnaires.12 In the original questionnaire, the Pearson's correlation coefficient between the daytime sleepiness domain of the QSQ and the Epworth test was −0.64, and in our case it is −0.59. Likewise, the daytime symptoms domain of the original QSQ and those referring to the physical function, vitality and physical role of the SF-36 were 0.35, 0.85 and 0.72, respectively. These values were very similar to those found in our study, which were 0.52, 0.78 and 0.71. In the same manner as in the original questionnaire, the emotions and social interaction domains presented a strong correlation with their homologous domains of the SF-36 questionnaire, with correlation coefficients of 0.52 and 0.48, respectively.
Regarding the predictive validity of the test, as we have shown in our study there is an observed worsening of the HRQL in patients who present severe SAHS compared with those who present either a mild or moderate degree of disease. This deterioration in the quality of life was observed in all the domains of the questionnaire, except in the social interaction domain. The interpretation of this situation could be based on the fact that the score obtained in the social interaction domain can often be influenced by other factors that also influence quality of life, such as depression, invalidating physical problems or even adverse episodes that alter the daily life of patients.
Retest reliability was studied using the intraclass correlation coefficient of each of the domains, which demonstrated either moderate or good agreement in each.
In the sensitivity to change analysis after CPAP treatment, statistically significant differences were observed in the daytime and nighttime symptoms domains while nearly clinically significant in the daytime sleepiness domain. Nevertheless, the clinical significance that the original publication stated was not reached.12 We believe that the explanation of this phenomenon is due to the fact that the patients included in the initial questionnaire validation presented more marked SAHS symptoms, especially greater hypersomnia (mean Epworth test=14), while in our series the patients were more paucisymptomatic (mean Epworth test=7.6). Therefore, the possibility for significant clinical improvement in the original group after CPAP was greater than that of the series of the present study. Thus, our results support the use of the QSQ survey after the application of CPAP treatment, especially in the analysis of the changes in patient symptoms.
As for the limitations of the study, the following should be mentioned: the sample size is relatively small compared with other larger studies that comparatively analyzed the capacity for change in patients who had complied with treatment and those who had not as well as the size effect.22 On the other hand, a new sleep study was not done in order to verify the disappearance of the apnea at the end of the study. Lastly, no information was collected about the side effects of the CPAP treatment.
In conclusion, our validation study indicates that the Spanish version of the QSQ is a valid tool for determining HRQL while providing adequate psychometric characteristics for its use in patients with SAHS. Likewise, the QSQ is sensitive to the changes induced by treatment in the domains referring to SAHS symptoms.
FundingGrants were received from the Valencia Pulmonology Foundation (Fundación Valenciana de Neumología, 2008) and Gasmedi 2000 (2008).
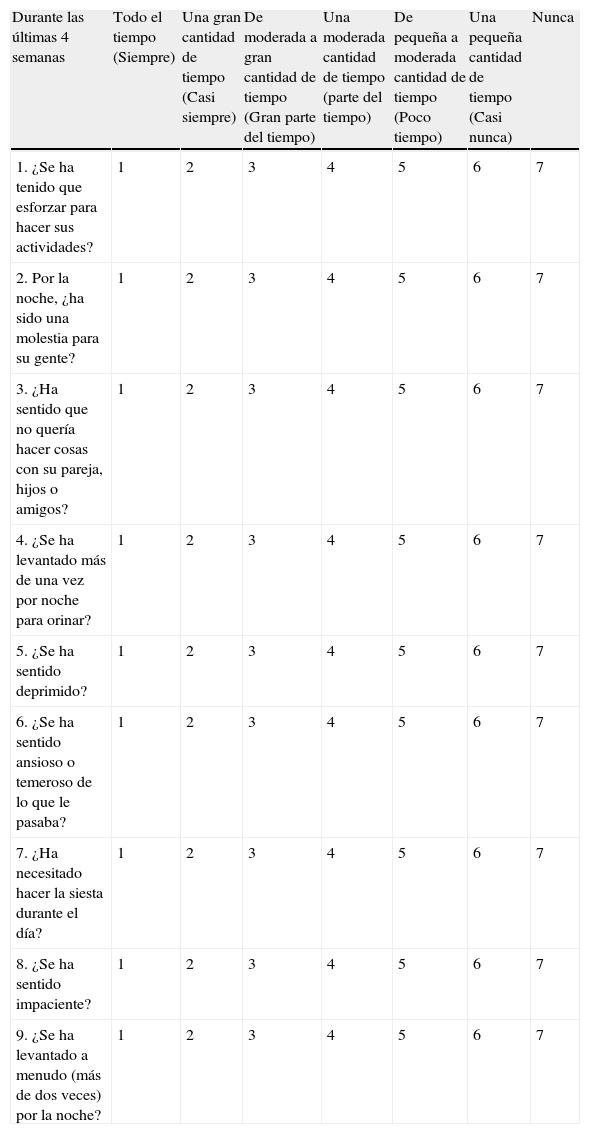
Este cuestionario ha sido diseñado para averiguar cómo le ha ido y cómo se ha sentido en las últimas cuatro semanas. Se le preguntará sobre el impacto que la apnea del sueño pudo haber causado en sus actividades de la vida diaria, su funcionamiento emocional y sus relaciones sociales, y sobre cualquier síntoma que ello pudiera haber causado.
| Durante las últimas 4 semanas | Todo el tiempo (Siempre) | Una gran cantidad de tiempo (Casi siempre) | De moderada a gran cantidad de tiempo (Gran parte del tiempo) | Una moderada cantidad de tiempo (parte del tiempo) | De pequeña a moderada cantidad de tiempo (Poco tiempo) | Una pequeña cantidad de tiempo (Casi nunca) | Nunca |
| 1. ¿Se ha tenido que esforzar para hacer sus actividades? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 2. Por la noche, ¿ha sido una molestia para su gente? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 3. ¿Ha sentido que no quería hacer cosas con su pareja, hijos o amigos? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 4. ¿Se ha levantado más de una vez por noche para orinar? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 5. ¿Se ha sentido deprimido? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 6. ¿Se ha sentido ansioso o temeroso de lo que le pasaba? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 7. ¿Ha necesitado hacer la siesta durante el día? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 8. ¿Se ha sentido impaciente? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 9. ¿Se ha levantado a menudo (más de dos veces) por la noche? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Durante las últimas 4 semanas | Muchísimo/a | Mucho/a | Bastante | Algo/Alguna | Poco/a | Muy poco/a | Nada |
| 10. ¿Ha tenido dificultad en intentar recordar cosas? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 11. ¿Ha tenido dificultad en intentar concentrarse? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 12. ¿Se ha sentido malhumorado cuando le han dicho que sus ronquidos eran molestos o irritantes? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 13. ¿Se ha sentido culpable en su relación con los miembros de la familia o amigos cercanos? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 14. ¿Ha percibido un descenso en el rendimiento de su trabajo? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 15. ¿Se ha preocupado por problemas de corazón o muerte prematura? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Durante las últimas 4 semanas, cuánto problema ha tenido con: | Un problema muy grande | Un gran problema | Entre mediano y gran problema | Un problema mediano | Entre mediano y pequeño problema | Un pequeño problema | Ningún problema |
| 16. Tener que luchar para permanecer despierto durante el día | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 17. Sentir que disminuía su energía | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 18. Sentir fatiga excesiva | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 19. Sentir que las actividades habituales requieren un esfuerzo extra para realizarlas o completarlas | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 20. Dormirse si no estaba estimulado o activo | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 21. Dificultad por tener la boca/garganta seca o dolorida al despertar | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 22. La dificultad en volver a dormirse si se despierta por la noche | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 23. Sentir que pierde energía | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 24. Preocuparse por las veces que deja de respirar por la noche | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 25. Roncar fuerte | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 26. Dificultades con la atención | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 27. Dormirse de repente | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 28. Despertarse por la noche con sensación de ahogo | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 29. Despertarse por la mañana cansado | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 30. Sensación de que su sueño no es reparador | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 31. Dificultad en permanecer despierto mientras lee | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 32. Luchar contra la necesidad de dormirse mientras conduce | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
Please cite this article as: Catalán P, et al. Consistencia interna y validez de la versión española del cuestionario de calidad de vida específico para el síndrome de apneas-hipopneas del sueño Quebec Sleep Questionnaire. Arch Bronconeumol. 2012;48:107–13.
Copyright of the English version of the Quebec Sleep Questionnaire: Lacasse Y, Sériès F and Centre de Recherche, Centre de Pneumologie, Hôpital Laval, Institut Universitaire de Cardiologie et de Pneumologie de l’Université Laval, Québec, Canada. Yves.Lacasse@med.ulaval.ca.