Chronic respiratory patients (CRP) are either more susceptible to develop infections, or at increased risk of serious complications, hospitalization, long term use of drugs to treat infection, and death.
Vaccination is still the best preventing method to reduce the burden of lower respiratory tract infections, especially pneumonia, in this set of patients. Recommendations by the World Health Organization (WHO) for Influenza vaccination (IV) include CRP, regardless of the age.1 For pneumococcal vaccination (PV), WHO position statement prioritizes high coverage of infants; the benefits in adults are highlighted, and studies have shown that the 13-valent conjugate vaccine (PCV13) vaccination in the elderly can induce an immune response against vaccine serotypes that is as good as or better than 23-valent polysaccharide vaccine (PPV23). PCV13 is safe and effective in preventing non-invasive and invasive pneumococcal pneumonia.2–4
Each country adapts these recommendations based on local epidemiological trends and social and financial circumstances. In our clinical scenario IV is recommended for CRP with asthma under inhaled or systemic corticotherapy, chronic obstructive lung disease (COPD), cystic fibrosis, interstitial lung fibrosis, pneumoconiosis and bronchopulmonary dysplasia with a cost ranging from 5.84 to 6.05 EUR (with 37% reimbursement upon medical prescription). However, it is only provided by the national health service for those with cystic fibrosis, alpha-1 antitrypsin deficiency under replacement therapy, interstitial lung disease (ILD) under immunosuppressive therapy, neuromuscular diseases, and for all inhabitants aged 65 or above. Also in our clinical context PV has an average cost of 59.11 EUR (also with 37% reimbursement) and is recommended for those with chronic respiratory insufficiency, COPD, emphysema, asthma under chronic inhaled or systemic corticotherapy, bronchiectasis, ILD, cystic fibrosis, pneumoconiosis, neuromuscular diseases, pulmonary arterial hypertension, active neoplastic disease, transplant receptor or in the waiting list, and under immunosuppression.
The aim of our study was to ascertain the level of IV and PV coverage and its determinants, as well as to identify the main reasons behind adherence to vaccination. To do so, we conducted a cross-sectional study based of the fulfillment of a questionnaire distributed to all CRP that attended our pulmonary outpatient clinic during nearly 5 months (including the influenza vaccine season).
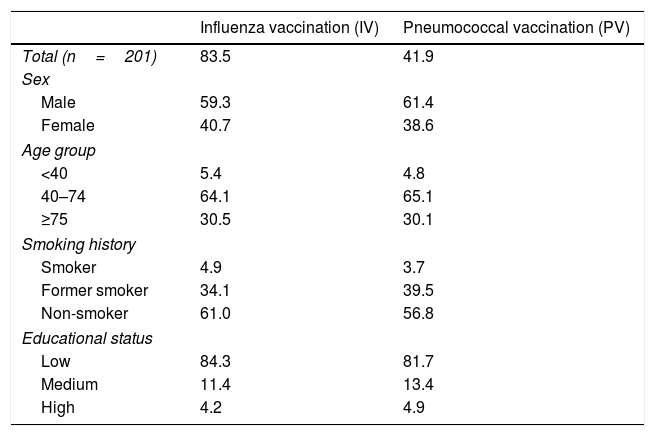
Of the total of 1362 patients that attended our clinic in the study period, 201 patients (14.75%) answered the questionnaire: 59.7% were men, with mean age of 65.3 years (SD=15.3); 8% were current and 36% former smokers; 81.8% had low vs. 13.1% with medium and 5.1% with high educational status. Major diagnosis registered were asthma (31.3%) followed by COPD (28.9%), ILD (23.4%) and lung cancer (1.0%). The overall coverage for IV and PV was 83.5% and 41.9%, respectively. For IV, 65.9% were vaccinated that present season, 63.5% in the previous one and 53.9% in any other season; 60.4% had had been vaccinated in the last two seasons. Among patients who received PV, 75.6% have been vaccinated with PCV13 and 35.4% with PPV23. Motivation towards vaccination was mainly due to previous medical recommendation (96.6% for IV and 96.1% for PV), followed by communication media (3.4% for IV and 3.9% for PV) and relatives’ counseling (1.4% and 0.8% for IV and PV, respectively). The chief hindrances towards vaccination were risk concerns (54.3%) and the possibility of acquiring flu regardless of vaccination (28.6%) for IV; economical concerns (39.3%), followed by risk concerns (25.0%), fear of injections/needles (8.9%) and difficult access to health care (5.4%) for PV. Vaccination rates according to determinants in CRP are showed in Table 1.
Vaccination rates (%).
| Influenza vaccination (IV) | Pneumococcal vaccination (PV) | |
|---|---|---|
| Total (n=201) | 83.5 | 41.9 |
| Sex | ||
| Male | 59.3 | 61.4 |
| Female | 40.7 | 38.6 |
| Age group | ||
| <40 | 5.4 | 4.8 |
| 40–74 | 64.1 | 65.1 |
| ≥75 | 30.5 | 30.1 |
| Smoking history | ||
| Smoker | 4.9 | 3.7 |
| Former smoker | 34.1 | 39.5 |
| Non-smoker | 61.0 | 56.8 |
| Educational status | ||
| Low | 84.3 | 81.7 |
| Medium | 11.4 | 13.4 |
| High | 4.2 | 4.9 |
Using a multivariable binary logistic regression model with forward elimination method, IV coverage was independently associated with age (p<0.006): patients aged ≥75 or 40–75 years were more likely to have IV coverage than patients aged <40 years (OR=9.28; 95%CI, 2.09–41.26; p=0.003 and OR=6.98; 95%CI, 1.92–25.35; p=0.003, respectively); smoking history (p=0.002): current and former smokers were less likely to have IV coverage than non-smokers (OR=0.09; 95%CI, 0.02–0.40; p=0.002 and OR=0.14; 95%CI, 0.04–0.50; p=0.003, respectively); and gender: women were less likely to have IV coverage than men (OR=0.28; 95%CI, 0.09–0.90; p=0.032). For PV coverage no factors were significantly associated.
Comparing to European statistics, where IV coverage in chronic medical conditions has a median rate of 50.3%5 and no data is available for PV coverage in adults, these results are above average for IV coverage mainly in elder CRP (where IV tends to be free of charge regardless of the clinical condition for people above 64 years). Nevertheless, both IV coverage in younger patients and overall PV coverage were considerably low. The vaccination rate and the educational status are in agreement with the most prevalent educational level of our population, unveiling a possible selection bias, but also portraying our reality. Further education on IV could be of benefit to resolve the main hindrance pointed out as the reason towards non-vaccination; reimbursement issues should be discussed insofar PV non-vaccination, since already 21 European member states offer one of the 2 conjugated pneumococcal vaccines for people 50 years of age and above and/or for risk groups in certain age groups.3
This is the starting point of a prospective cohort study currently underway to evaluate the cost-effectiveness of both vaccines, specially pneumococcal, and to ascertain their impact on the number of infectious intercurrences, use of antibiotics (which and how long), hospitalization and deaths.
We expect, given our current data and possibly with the results of our future investigation, to increase the awareness of local physicians and administrative boards to this issue in order to develop strategies to re-enforce vaccination in CRP.