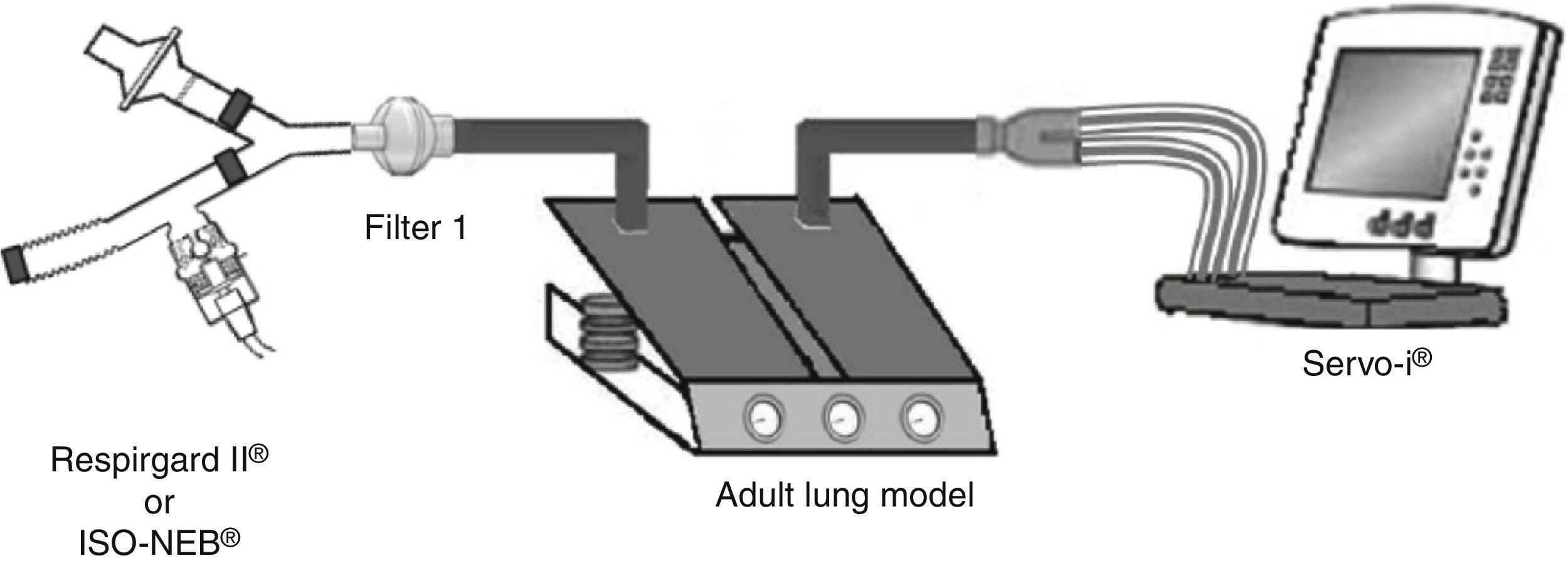
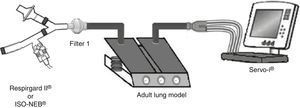
Pneumocystis jirovecii pneumonia (PJP) represents a significant cause of morbidity and mortality in immunosuppressed patients.1,2 Pentamidine, used in secondary prevention of PJP, is administered via inhalation and requires a specific nebulizer.3,4 In the recent ERS/ISAM Task Force Consensus Statement, the RespirgardII® (Vital Signs) was considered as the reference nebulizer to deliver pentamidine.5 Nebulizers with comparable properties are required because RespirgardII® is no longer available since its recent withdrawal from the market in some countries.3 Most of the nebulizers compared previously to the RespirgardII were ultrasonic nebulizers.6 In this study, we compared a jet nebulizer (ISO-NEB®, Teleflex) to RespirgardII® for pentamidine delivery. Both nebulizers possess one-way valves on the inspiratory and on the expiratory way and an expiratory filter as recommended.3,4 Both were driven by a similar air flow (8L/min) and deliver particles with similar size (MMAD: 1–2μm).3,7,8 In vitro, nebulizers were connected to a dual chamber lung model (5600i Dual Adult Test Lung®, Michigan Instrument Inc.) driven by a ventilator (SERVO-I®, Maquet) in volume-controlled mode simulating an adult breathing pattern (Vt=500mL; RF=15 breaths/min; I/E ratio=1:2; no end-inspiratory pause) (Fig. 1). Artificial lung compliance and resistance were set to 70mL/cmH2O and 5cmH2O, respectively. Nebulizations of a pentamidine solution (300mg/6mL sterile water) were performed in triplicate for each model in accordance with manufacturer's and guidelines recommendations until one minute after the appearance of the sputtering point.1,2,7,9 Inhaled dose, expressed in percentage of the nominal dose (ND), corresponding to the nebulized doses deposited on the filter interposed between nebulizer and lung model (weighed before the nebulization and after drying for 24h) multiplied by the relative mass of pentamidine. The residual volume was also quantified.
In the in vivo part, after ethical approval (2013/27JUI/375) and registration of the trial (NCT02277808), five non-smoker healthy male volunteers were recruited and signed a written inform consent. Each subject performed a spirometry according to the ATS/ERS guidelines.10 This was a randomized cross-over study based on CONSORT statement for clinical trials. Nebulizations of amikacin sulfate (Amukin®, Bristol-Myers Squibb) dissolved in 4mL of normal saline (125mg/mL) were made during 10min with both devices. During nebulization, tidal volume (Vt; L), respiratory frequency (RF; min−1) and minute ventilation (VE; Lmin−1) were monitored by inductance plethysmography (Respitraces®, Ambulatory Monitoring Inc.). Participants were requested (1) to empty their bladder before nebulization, (2) to inhale spontaneously through the mouthpiece with a nose clip in a sitting position, (3) to collect their urine for 24h following nebulization and (4) to observe a wash-out period of one week between both nebulizations. Then comparison was performed by sampling the daily urinary excretion of nebulized amikacin following the technique previously described by Dequin et al.11 and analyzed by High Performance Liquid Chromatography. The total daily amount of amikacin excreted in the urine (Cu max) was calculated from cumulating amikacin amount measured at each micturition (Cu) and represents the lung dose. The elimination constant (Ke) was calculated from the fitted curve of the cumulated amount of amikacin excreted in the urine plotted versus the time. The equation is Cu=Cu max(1−e−Ket).
Inhaled dose was similar between devices, 28.7% (22.7; 33.5) vs 29.3% of ND (26.3; 33.1) for ISO-NEB® and for RespirgardII® (p=0.792). Residual volume was 0.9313g (0.9270; 0.9382) and 1.4087g (1.3845; 1.4416) for ISO-NEB® and for RespirgardII®.
In vivo, all volunteers (23.5+/−1.3 years) had spirometric values in the normal range. Cu max was similar between devices with 3.5% (3.1; 3.6) and 3.6% of ND (2.2; 4.2), (p=0.893) for ISO-NEB® and RespirgardII®, respectively. Urine volume was 1.37L (0.80; 1.72) and 1.30L (0.75; 2.10) for ISO-NEB® and RespirgardII®, respectively (p=0.686). Elimination constant (Ke) of the drug following nebulization was similar for both devices (0.159 (0.078; 0.208) vs 0.130 (0.085; 0.162) for ISO-NEB® and for RespirgardII®, respectively (p=0.225)). There was no significant difference in RF, Vt and VE. Our results were in the range of previous studies even if the comparison is difficult because there were no data for ISO-NEB® and the previous studies on RespirgardII® presented many differences in protocols and measurements techniques.6,9,12,13 We used the most frequent dosage reported in the previous studies and in the manufacturer's recommendations (300mg pentamidine in 6mL sterile water).1,2,7,9 The airflow rate (8Lmin−1) to produce aerosolized pentamidine was in the range of the rate described in previous studies about RespirgardII® (about 6–10Lmin−1 for RespirgardII®) or recommended by the manufacturer for ISO-NEB® (from 5 to 9Lmin−1). The total amount of drug reaching the lungs was similar and lower than 5% of ND for both devices. Our results are in line with those obtained with pentamidine in prior studies (2–6.74% of ND).4,6,9,12 Some methodological conditions need to be discussed. In vitro, we used the residual gravimetric technique even though it was not validated for pentamidine. However previous studies validated this process for different drugs.14 In vivo, we used amikacin sulfate because pentamidine has not been previously considered as a valid pharmacological marker of pulmonary deposition.11,15 For a methodological consideration, we recruited only male subjects because it is easier for a man to collect his urine without loss and to prevent potential fetal risk ototoxicity in pregnant female subjects. Finally, we did not measure the particle sizes for both nebulizers, but according to previous studies and manufacturer's data we can consider that they were similar (1–2μm). It is important to notice that the two nebulizers are in the same price range. In conclusion, this in vitro and in vivo study demonstrated that ISO-NEB® and RespirgardII® have similar properties in the conditions study. Further clinical studies are needed to confirm that ISO-NEB® is a valuable alternative to the reference nebulizer recommended by guidelines for pentamidine delivery. Altogether these data suggest that the performance of both devices is similar in the conditions of this in vitro and in vivo study.