Histoplasmosis is an infection caused by Histoplasma capsulatum, a dimorphic fungus endemic to the Americas and parts of Africa and Asia. In Europe, it primarily occurs as an imported infection, although native cases have been reported in Italy and Turkey.1 In recent years, due to increased travel to endemic areas and immigration, the diagnosis of histoplasmosis in non-endemic areas has increased, including in Spain.2 Imported cases occur mainly in young adults, and very rarely in children or adolescents. In a European registry,1 only 4 of 118 cases were diagnosed in individuals under the age of 20 years. We report the case of an immunocompetent teenager presenting with acute pulmonary histoplasmosis after a trip to Mexico.
This was a previously healthy 15-year-old male patient with a complete childhood vaccination schedule, who consulted due to a 9-day history of fever of up to 39.5°C. He had returned 20 days previously from a 10-day holiday in Riviera Maya. He presented asthenia, hyporexia, generalized myalgia, headache, and mild cough without rhinorrhea. Physical examination was normal. The blood test showed discretely elevated acute phase reactants (CRP 2.8mg/dL), with no other abnormalities, and a blood culture was sterile.
The study was expanded to include serology (Epstein–Barr virus, cytomegalovirus, hepatitis A and B, HIV, Toxoplasma, Entamoeba histolytica, Brucella, Borrelia, dengue, parvovirus B19, Echinococcus, Leishmania, syphilis, Legionella, and Mycoplasma pneumoniae), which was negative; malaria antigens, thick blood smear, QuantiFERON®, and cultures of blood, pharyngeal exudate, and urine were negative; viral culture of nasopharyngeal exudate was negative; Mantoux test 0mm; peripheral blood smear normal. Zika virus serology was IgG+/IgM−, and Zika virus PCR in blood was negative. Endemic fungi serologies (Blastomyces, Coccidioides, Histoplasma, Paracoccidioides) were negative. The abdominal ultrasound was significant for splenomegaly 15.6cm, and chest X-ray showed an increase in density in the right paracardiac region, consistent with pneumonic consolidation. Empiric treatment began with amoxicillin.
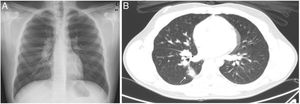
Due to the persistence of fever (total of 14 days), the patient was evaluated in cardiology (ECG and echocardiography) and ophthalmology (fundus), but no changes were observed. In addition to the initial symptoms, he developed profuse sweating mainly at night and a weight loss of 3kg. Three weeks after the onset of the syndrome, endemic fungi serologies were repeated; chest X-ray showed bilateral and paratracheal hilar adenopathies (Fig. 1A), and abdominal ultrasound revealed splenomegaly increased to 17cm and lymphadenopathies in the hilar region. A chest-abdominal computed tomography was performed (Fig. 1B), which showed a right parenchymal nodule, and bulky ipsilateral hilar and mediastinal lymphadenopathies (right paratracheal, aortopulmonar, subcarinal, and azygo-esophageal recess). In view of the possibility of a lymphoproliferative syndrome, bronchoalveloar lavage and bone marrow aspirate were performed 29 days after the onset of symptoms. The histopathological studies of both samples were normal, with no isolates on bacterial and fungal cultures, and negative panfungal PCR (18S rRNA) and negative specific PCR for Aspergillus and Mycobacterium tuberculosis. Finally, seroconversion of Histoplasma antibodies was observed on immunodiffusion in a second serum sample.
A diagnosis of acute histoplasmosis was made, and in due to the persistence of symptoms for more than 4 weeks, treatment with itraconazole was administered for 6 weeks. In the successive follow-up visits, the patient showed complete resolution of symptoms, along with normalization of the radiographic changes.
To our knowledge, this is the first case of pediatric histoplasmosis published in Spain. The infection occurs by inhalation of conidia during activities that involve contact with contaminated surfaces. Severity depends on the intensity of exposure and the host immune status, and the clinical spectrum is broad. Most immunocompetent cases remain asymptomatic. Symptomatic acute forms in immunocompetent patients usually consist of a flu-like illness, with fever and cough, which is self-limited to 2 weeks.3 The radiological presentation is highly unspecific, with diffuse reticulonodular infiltrates and hilar and mediastinal lymphadenopathies. The most severe form is a progressive disseminated disease, which can occur after acute infection or reactivation of a past infection. It is caused by hematogenous spread of the fungus in immunocompromised individuals or at extreme ages of life.
In non-endemic areas, the infection may be seen in 2 types of populations with different clinical profiles: travelers and immigrants from endemic areas.4 In Spain, most are from Central and South America.5 In the case of travelers, it is common to identify risk activities that involve exposure to the fungus (caving, construction, excavations, etc.). Subjects tend to be immunocompetent and, if they are symptomatic, the disease presents as an acute pulmonary infection starting within 2 months of exposure, that is not usually severe. It can occur in a cluster of several cases.6 Our patient reported having made a tour to underground lakes during his trip, which could explain the risk exposure.
Diagnostic confirmation is obtained by isolating the fungus, although this is very rare in the acute forms. In these cases, microbiological diagnosis is usually based on serological testing. Histoplasma antibodies begin to be detectable 2–4 weeks after infection, reaching a sensitivity of 90%,7 so in suspected cases with a first negative serology, such as ours, it is advisable to repeat the test later. Other diagnostic tests include histopathological studies, antigen detection (not available outside the US) or, more recently, PCR, available only in reference laboratories. Treatment depends on the clinical form, severity, and immune status of the individual. The Infectious Diseases Society of America (IDSA) recommends treatment in acute moderate or severe pulmonary forms and in mild forms when symptoms persist for more than 4 weeks.8
A diagnosis of histoplasmosis should be considered in travelers returning from endemic areas with fever and respiratory symptoms who have participated in risk activities, or after other companions are diagnosed. Due to its self-limited course, this infection is probably underestimated in non-endemic areas, so a high index of suspicion is required.
Please cite this article as: García Mancebo J, Aguilera-Alonso D, Rincón-López EM, Navarro ML. Histoplasmosis aguda importada en un adolescente con sospecha de síndrome linfoproliferativo. Arch Bronconeumol. 2019;55:341–343.