Glatiramer acetate (GA) is a synthetic copolymer-1 approved as first-line treatment for relapsing-remitting multiple sclerosis (RRMS). Several studies have indicated that GA modulates different levels of the immune response, but no specific warnings regarding its use and potential reactivation of latent tuberculosis (TB) infection exist.1
A 37-year-old man, engineer, active smoker (10 pack-years), with a 6-year history of RRMS but otherwise healthy, attended our pulmonary clinic reporting a 1-week history of productive cough, fever, left side pleuritic chest pain, and malaise. He had been taking daily subcutaneous GA since his RRMS diagnosis, but no other concomitant medication. Relevant past medical history included a 9-month course of isoniazid as primary chemoprophylaxis for TB 16 years previously, after a positive tuberculin skin test as part of a TB contact study. He denied any other subsequent known contacts or exposure to high-risk environments. His physical exam was normal and blood tests showed a mild elevation of acute phase reactants. A chest computed tomography revealed left upper lobe alveolar infiltrate and a 1.8cm lung cavity. The sputum smear was positive for acid-fast bacilli and empiric 4-drug treatment was started. Sputum culture confirmed Mycobacterium tuberculosis and the patient recovered completely after 6 months of treatment.
Peripheral immunomodulatory mechanisms of GA include: binding to major histocompatiblity class II molecules, alteration of the innate immune response, T-cell receptor antagonism, T-cell deviation and modification of B-cells.1 It has an inhibitory effect on monocyte reactivity, modifying dendritic cells and monocytes secretion to produce less tumor necrosis factor (TNF)-α and interleukin (IL)-12, and more IL-10 and transforming growth factor (TGF)-β. It stimulates T-helper (Th)-2 anti-inflammatory response, in detriment of Th1 pro-inflammatory effects, with subsequent decrease of interferon (IFN)-γ levels.
The initial defense against TB infection involves alveolar macrophages. Although there is a role for many types of T-lymphocytes, the major effector cell in cell-mediated immunity in TB is the helper T-cell. Studies have shown that patients with active TB have mainly a Th2-type response, whereas those with latent disease show a Th1-type response. The strength of the latter relates directly to the clinical manifestations of the disease, where low levels of circulating IFN-γ in peripheral blood are associated with severe clinical TB and advanced disease.3 Inhibition of TNF-α and reduced levels of IL-12, both effects of GA treatment, are associated with increased risk of mycobacterial disease. GA also affects macrophage activation, critical in the elimination of mycobacteria, as increased levels of IL-10 and TGF-β and reduced levels of IFN-γ are associated with macrophage inactivation and activation, respectively.3
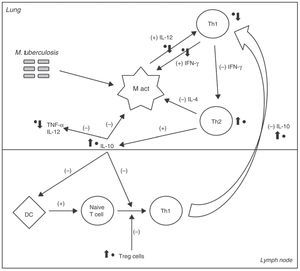
Fig. 1 illustrates the different sites where GA would intervene in the immune response to M. tuberculosis. These effects are likely to induce an imbalance between the pro- and anti-inflammatory factors that control TB infection, facilitating disease reactivation.2
A simplified scheme of the cellular immune response to M. tuberculosis. The (+) and (−) signs indicate the positive or negative feedback induced by each cell or cytokine under normal circumstances. The main sites where glatiramer acetate (GA) directly participates are marked with a black dot (•) and arrow to indicate the induced effect (increase or decrease). It directly affects macrophage activation (M act) by inhibiting T-helper 1 (Th1) response and thus, reducing levels of circulating interferon (IFN) γ, which plays an important role in their activation. It inhibits production of interleukin (IL) 12 which amplifies the Th1 response.3 The IL-10 production is increased, directly inhibiting macrophage activation, and blocking the effects of dendritic cells (DC) and the differentiation of naive T cells into Th1 cells.4 It blocks the migration of Th1 cells from lymph nodes back to the lungs, and inhibits the expression and release of tumor necrosis factor (TNF)-α.4 Finally, it stimulates T-regulatory (T-reg) cells which also block the Th1 response.4
To the best of our knowledge this is the first documented case of pulmonary TB reactivation in a patient receiving GA treatment. Screening for latent TB infection may be an important first step before starting GA in patients with RRMS.
FundingNo funding was received.
Please cite this article as: Sanchez-Salcedo P, de-Torres JP. Efectos inmunomoduladores del glatirámero acetato y su potencial papel en la reactivación de la tuberculosis pulmonar. Arch Bronconeumol. 2015;51:656–657.