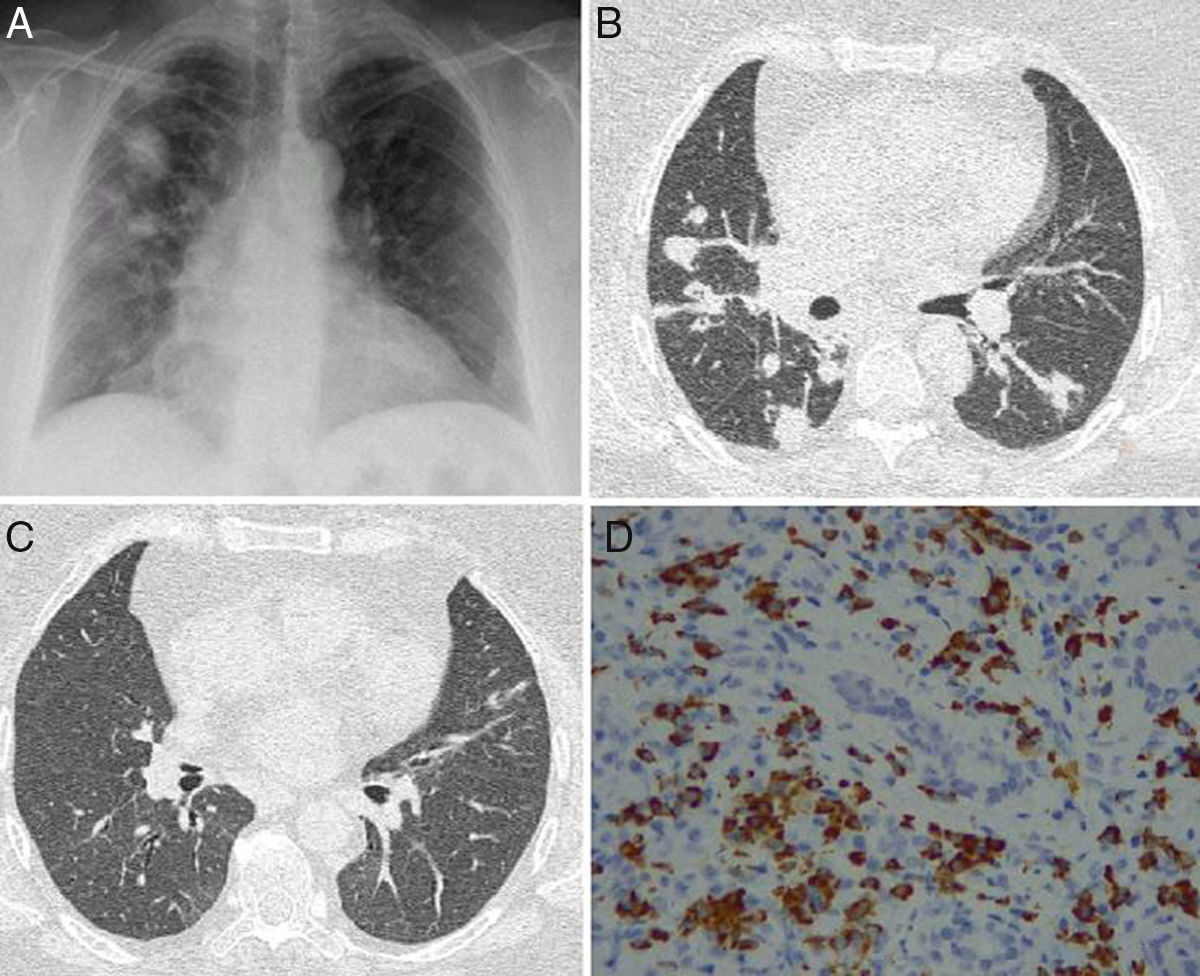
We report the case of a 52-year-old woman, no significant medical history, who presented in the emergency room with a 2-month history of cough with whitish expectoration, asthenia, and low-grade fever. A chest X-ray was performed, which showed bilateral pseudonodular consolidations of varying sizes, with poorly defined borders, mainly in the right hemithorax and upper fields (Fig. 1A). An infectious process was first suspected, and the patient received various courses of antibiotics as an outpatient, but failed to respond. The patient worsened over the next few days: respiratory failure developed and nodules and consolidations in bands were observed on computed tomography (CT) (Fig. 1B and C). On physical examination, slightly painful masses were seen on both eyelids, with no signs of inflammation and no dry eye syndrome. Tumor infiltration was ruled out by biopsy. Bronchoscopy was performed with transbronchial biopsy that revealed collapsed lung parenchyma with small lymphocytic aggregates, bronchoalveolar lavage containing 76% histiocytes, 8% segmented cells, and 16% lymphocytes; lymphocyte populations consisted of 67% T cells (CD4+64%, CD8+26%), 11% NK cells, and 22% B cells. Bronchial aspirate microbiology was negative. Complete blood count was normal, with no eosinophilia and normal immunoglobulin (Ig) IgE, C3 and C4 values. However, ANCA at a titer of 1/180 with a cytoplasmic pattern and ANCA-PR3 (82.50U/ml) were both positive. Antinuclear antibodies (ANA) and MPO-ANCA were negative. Raised serum IgG was detected (1700mg/dl) mainly due to elevated IgG4 subclass (157mg/dl) and a slight increase in IgG3. IgG4-related disease (IgG4-RD) was suspected, so the pathology study of the lacrimal gland was reviewed, and specific immunohistochemistry techniques demonstrated interstitial inflammatory infiltrate with lymphocytes, macrophages, and plasma cells expressing cytoplasmic positivity for IgG4 (Fig. 1D).
(A) Chest X-ray posteroanterior image with consolidations predominantly in the right side. (B) Axial CT showing masses and nodules associated with the consolidations. (C) Axial CT of the chest showing resolution of pulmonary lesions after corticoid therapy. (D) Lacrimal gland tissue showing interstitial inflammatory infiltrate caused by lymphocytes, macrophages, and plasma cells with cytoplasmic positivity for IgG4 (IgG4/IgG>40%).
Treatment started with methylprednisolone at a dose of 1mg/kg, and clinical and radiological improvement was achieved within a few days. After 2 months, the patient was asymptomatic with complete radiological resolution, so we began to taper the steroid dose. At that time, lung function tests showed a moderate restrictive change and reduced diffusing capacity of the lung for CO (DLCO). Two months later, she was re-admitted for sudden-onset dyspnea due to pulmonary thromboembolism with hemodynamic repercussions that required fibrinolysis, and deep vein thrombosis of the lower limbs. The thrombophilia study was negative. After a year and a half of follow-up, the corticosteroid dose was reduced and she developed an exacerbation with recurrence of the cough and lung lesions, which were controlled after the corticosteroid dose was increased again. She has subsequently remained asymptomatic on a maintenance dose of 5mg/day of prednisone.
IgG4-RD is a recently described entity. Its prevalence in our setting is unknown, as the available epidemiological data come from Asian populations.1 IgG4 is the least abundant immunoglobulin in serum (<5%).2 Its structure is similar to that of other antibodies, but it has inefficient disulfide bonds between the chains that allows them to separate and form new bonds with other fragments of IgG4. This process generates divalent molecules that can form new immune complexes that have little ability to activate complement, so this immunoglobulin is believed to have anti-inflammatory properties. However, IgG4 can be elevated in some autoimmune diseases, and it is postulated that cytokines and B cell infiltration develop as an immune response induced by an unknown trigger, leading to the production of IgG4-secreting plasma cells and overexpression of transforming growth factor β (TGF-β), which has a recognized capacity to promote tissue fibrosis.
IgG4-RD includes different entities that affect one or more organs synchronously or metachronously,3 traditionally defined with their own nomenclature4 as fibrosing thyroiditis (Riedel's thyroiditis), retroperitoneal fibrosis (Ormond's disease), autoimmune pancreatitis, and Mikulicz's disease, etc. Clinical suspicion is essential for diagnosis, as the initial clinical picture can be nonspecific and heterogeneous, and the patient may be referred to many different consultants, delaying the diagnosis that will finally be reached by combining clinical criteria and laboratory and histological findings.5 The difficulty increases if we take into account that 16% of patients show spuriously normal IgG4 levels,6 and that raised IgE7 and peripheral eosinophilia may also be encountered in up to 25%.8 Airway, interstitial, pleural effusion, or mediastinal lung involvement9,10 occurs in 14% of cases but involvement of the pancreas, lacrimal and salivary glands, and kidney are more common. It is important to consider that IgG4-RD may also be associated with other autoimmune diseases and malignancies,11 and some cases have resolved spontaneously.12 In the differential diagnosis of lung involvement, we must consider cancer, infections, and interstitial diseases. Our patient's chest CT scan suggested lepidic adenocarcinoma, due to findings of peribronchovascular consolidations and multiple bilateral pulmonary nodules with right paratracheal and left supraclavicular lymphadenopathies. Finally, the IgG4 titer and pathology study were inconclusive.
Although IgG4-RD has not yet been described in association with thromboembolic disease, it has been associated with vascular inflammatory phenomena of the aorta and peripheral arteries.13 We believe, therefore, that there is an interesting possibility that this disease predisposes to vascular damage or induces a hypercoagulable state that would warrant special attention in the follow-up of patients and justify an update of the recommendations for thromboembolic prophylaxis.
Please cite this article as: Fernandez García D, León Fábregas M, Mancheño Franch N, Benavent Corai V. Enfermedad relacionada con IgG4 con afectación pulmonar. Arch Bronconeumol. 2019;55:165–166.